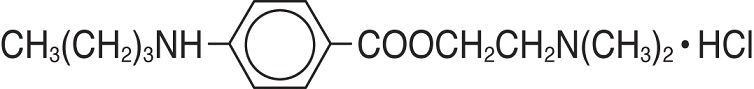Label: TETRACAINE HYDROCHLORIDE solution
- NDC Code(s): 70518-4183-0, 70518-4183-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0065-0741
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Tetracaine Hydrochloride Ophthalmic Solution 0.5% STERI‑UNIT - ®safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETetracaine Hydrochloride Ophthalmic Solution 0.5% is indicated for procedures requiring a rapid and short-acting topical ophthalmic anesthetic.
-
2 DOSAGE AND ADMINISTRATION2.1 Topical Administration - One drop topically in the eye as needed. Discard unused portion. 2.2 Sterile Field Administration - Open package using standard aseptic technique. The ...
-
3 DOSAGE FORMS AND STRENGTHSSterile ophthalmic solution containing 0.5% w/v tetracaine hydrochloride equivalent to tetracaine 0.44% w/v.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Corneal injury with Intracameral Use - Not for injection or intraocular use. Do not use intracamerally because use of TetracaineHydrochloride Ophthalmic Solution 0.5% may lead to damage of ...
-
6 ADVERSE REACTIONSThe following serious ocular adverse reactions are described elsewhere in the labeling: Corneal injury with Intracameral Use - [See - Warnings and Precautions (5.1)] Corneal ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies with Tetracaine Hydrochloride Ophthalmic Solution 0.5% in pregnant women. Animal developmental and ...
-
10 OVERDOSAGEProlonged use of a topical ocular anesthetic including TetracaineHydrochloride Ophthalmic Solution 0.5% may produce permanent corneal opacification and ulceration with accompanying visual loss ...
-
11 DESCRIPTIONTetracaine hydrochloride is chemically designated as benzoic acid, 4-(butylamino)-,2-(dimethylamino) ethyl ester, monohydrochloride. Its chemical formula is C - 15H - 24N - 2O - 2• HCl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tetracaine blocks sodium ion channels required for the initiation and conduction of neuronal impulses thereby affecting local anesthesia. 12.3 Pharmacokinetics - The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to assess the genotoxicity of tetracaine hydrochloride have not been reported in the published literature. Long-term animal ...
-
14 CLINICAL STUDIESTopical administration of tetracainehydrochloride ophthalmic solution results in localized temporary anesthesia. The maximum effect is achieved within 10–20 seconds after instillation, with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTetracaine Hydrochloride Ophthalmic Solution 0.5% STERI-UNITS® is supplied as single patient use, 4 mL filled in 4-mL natural medium- or low-density polyethylene plastic DROP-TAINER® dispensers ...
-
17 PATIENT COUNSELING INFORMATIONEye Care Precaution - Advise patients that, due to the effect of the anesthetic, their eyes will be insensitive up to 20 minutes and that care should be taken to avoid accidental injuries ...
-
PRINCIPAL DISPLAY PANELDRUG: Tetracaine Hydrochloride - GENERIC: tetracaine hydrochloride - DOSAGE: SOLUTION - ADMINSTRATION: OPHTHALMIC - NDC: 70518-4183-0 - NDC: 70518-4183-1 - PACKAGING: 4 mL in 1 BOTTLE, DROPPER - OUTER PACKAGING ...
-
INGREDIENTS AND APPEARANCEProduct Information