Label: TIZANIDINE tablet
- NDC Code(s): 60505-0251-1, 60505-0251-2, 60505-0251-3, 60505-0252-1, view more
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIZANIDINE TABLETS safely and effectively. See full prescribing information for TIZANIDINE TABLETS. TIZANIDINE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Tizanidine is indicated for the treatment of spasticity in adults.
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Evaluation and Testing Before and After Initiating Tizanidine - Monitoring of aminotransferase levels is recommended at baseline and 1 month after maximum dose is achieved [see ...
-
3 DOSAGE FORMS AND STRENGTHS Tablets - 2 mg - white to off-white, round, scored tablets, imprinted “APO” over “TI-2” on one side and plain with a bisect score on the other side. 4 mg- white to off-white, round, scored ...
-
4 CONTRAINDICATIONS Tizanidine is contraindicated in patients: • taking strong CYP1A2 inhibitors [see Drug Interactions (7.1)]. • with a history of hypersensitivity to tizanidine or the ingredients in tizanidine ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypotension - Tizanidine is an α2-adrenergic agonist that can produce hypotension[see Adverse Reactions (6.1) and Drug Interactions (7.5)]. Syncope has been reported in patients treated with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in other sections of the prescribing information: • Hypotension [see Warnings and Precautions (5.1)] • Liver Injury ...
-
7 DRUG INTERACTIONS 7.1 Strong CYP1A2 Inhibitors - Concomitant use of tizanidine with strong cytochrome P450 1A2 (CYP1A2) inhibitors (e.g., fluvoxamine, ciprofloxacin) is contraindicated. Changes in pharmacokinetics ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with use of tizanidine tablets in pregnant women. In animal studies, administration of tizanidine ...
-
9 DRUG ABUSE AND DEPENDENCE 9.1 Controlled Substance - Tizanidine tablets contain tizanidine, which is not a controlled substance. 9.2 Abuse - Abuse is the intentional, non-therapeutic use of a drug, even once, for its ...
-
10 OVERDOSAGE A review of the safety surveillance database revealed cases of intentional and accidental tizanidine overdose. Some of the cases resulted in fatality and many of the intentional overdoses were ...
-
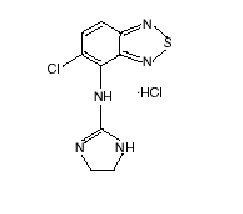
11 DESCRIPTION Tizanidine Tablets, USP contains tizanidine hydrochloride as the active ingredient, which is a central alpha2-adrenergic agonist. Its chemical name is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tizanidine is a central alpha-2-adrenergic receptor agonist and presumably reduces spasticity by increasing presynaptic inhibition of motor neurons. The effects of ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Tizanidine was administered to mice for 78 weeks at oral doses up to 16 mg/kg/day, which is 2 times the maximum ...
-
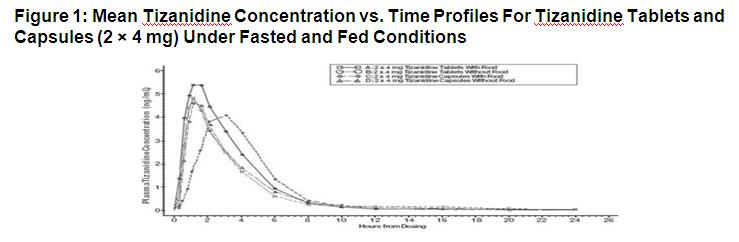
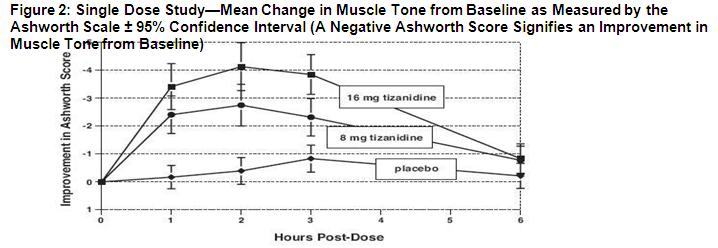
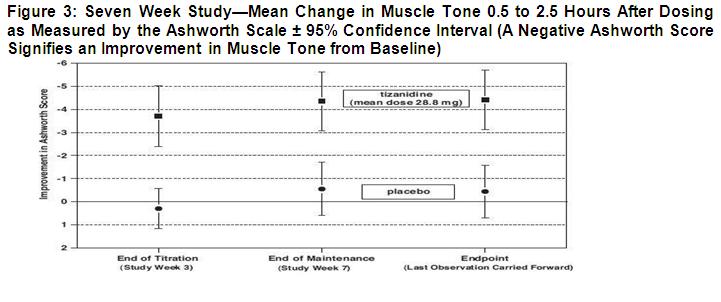
14 CLINICAL STUDIES The efficacy of tizanidine for the treatment of spasticity was demonstrated in two adequate and well-controlled studies in patients with multiple sclerosis or spinal cord injury (Studies 1 and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tizanidine Tablets, USP 2 mg are available for oral administration as white to off-white, round, scored tablets, imprinted “APO” over “TI-2” on one side and plain with a ...
-
17 PATIENT COUNSELING INFORMATIONSerious Drug Interactions - Advise patients they should not take tizanidine tablets if they are taking fluvoxamine or ciprofloxacin because of the increased risk of serious adverse reactions ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 2 MG BOTTLE LABEL - APOTEX CORP. NDC 60505-0251-3 - Tizanidine Tablets, USP - Equivalent to 2 mg tizanidine - 2 mg - Rx Only - 100 Tablets
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 4 MG LABEL - APOTEX CORP. NDC 60505-0252-3 - Tizanidine Tablets, USP - Equivalent to 4 mg tizanidine - 4 mg - Rx Only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information