Label: COLTALIN- acetaminophen, chlorpheniramine maleate, phenylephrine hydrochloride tablet
- NDC Code(s): 51467-005-01, 51467-005-02
- Packager: FORTUNE PHARMACAL COMPANY LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
■ temporarily relieves these symptoms due to a cold, the flu, or hay fever:
■ minor aches and pains
■ sore throat
■ headache
■ runny nose
■ sinus congestion and pressure
■ nasal congestion due to a cold or hay fever
■ runny nose
■ sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever
■ temporarily reduces fever
■ promotes nasal and/or sinus drainage -
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if:
■ adult takes more than 12 tablets in 24 hours, which the maximum daily amount for this product
■ child takes more than 5 doses in 24 hours
■ taken with other drugs containing acetaminophen
■ adult has 3 or more alcoholic drinks everyday while using this product -
DO NOT USE
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know whether a prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. -
ASK DOCTOR/PHARMACIST
Ask a doctor before use if the user has
■ heart disease
■ high blood pressure
■ diabetes
■ glaucoma
■ liver disease
■ thyroid disease
■ a high fever
■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate glandAsk a doctor or pharmacist before use if the user is
■ taking tranquilizers or sedatives
■ taking the blood thinning drug warfarin - WHEN USING
-
STOP USE
Stop use and ask a doctor if
■ pain or nasal congestion gets worse or lasts more than 5 days (children) or 7 days (adults)
■ sore throat persists for more than 2 days
■ fever gets worse or lasts more than 3 days
■ nasal congestion is accompanied by fever
■ nervousness, dizziness, or sleeplessness occur
■ redness or swelling is present
■ new symptoms occur
■ any of the following occurs (these could be signs of a serious condition):
■ severe sore throat
■ sore throat is accompanied or followed by fever, headache, rash, nausea or vomitingDo not give to children under 6 years of age unless directed by a doctor.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
■ adults and children 12 years of age and over: take 2 tablets every 4 hours, while symptoms persist, not to exceed 6 doses (12 tablets) in 24 hours, or as directed by a doctor
■ children 6 to under 12 years of age: take 1 tablet every 4 hours, while symptoms persist, not to exceed 5 doses (5 tablets) in 24 hours, or as directed by a doctor
■ children under 6 years of age : consult a doctor - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
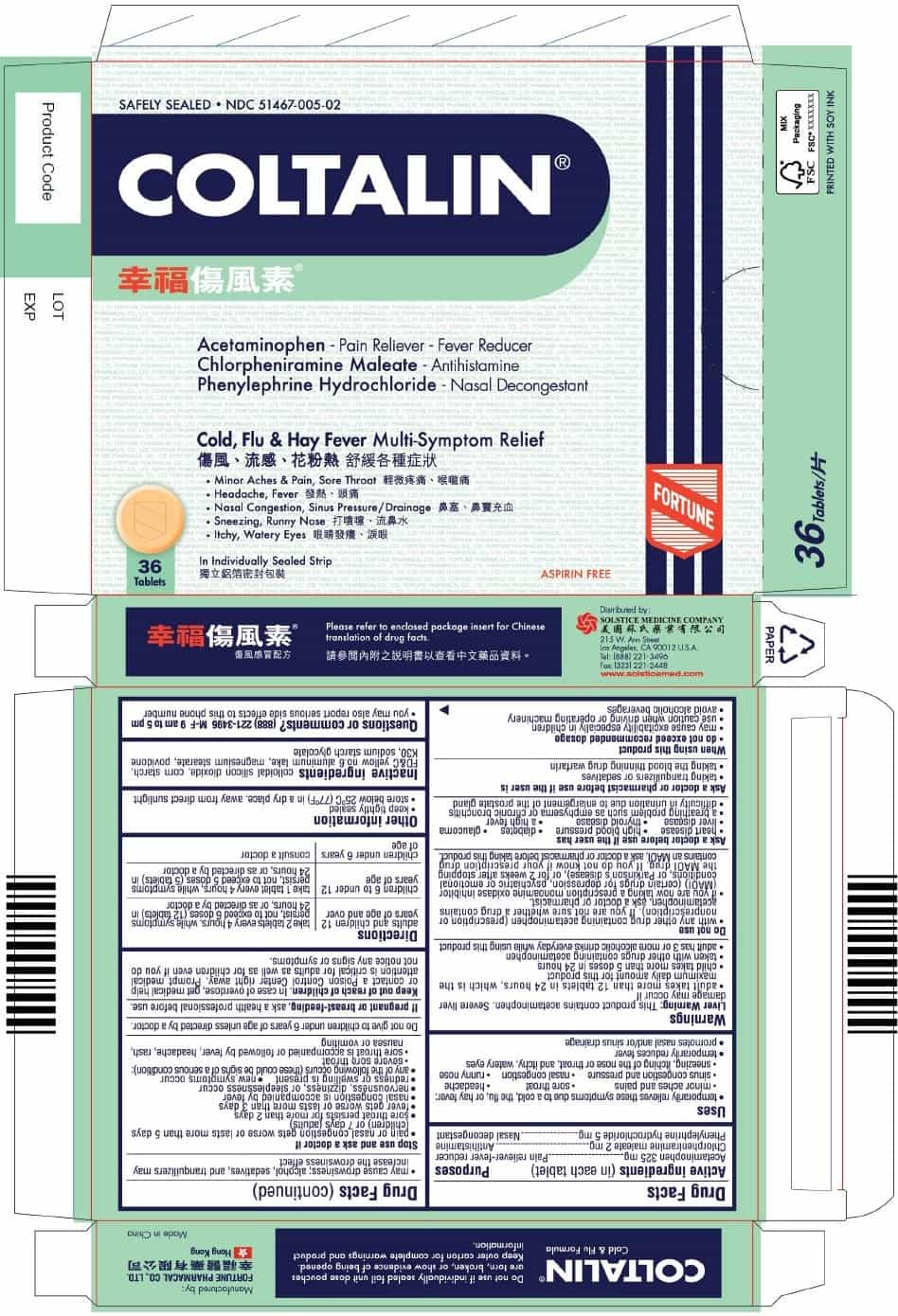
PRINCIPAL DISPLAY PANEL
COLTALIN
Safely Sealed
NDC 51467-005-01
Acetaminophen - Pain Reliever-Fever Reducer
Chlorpheniramine maleate - Antihistamine
Phenylephrine hydrochloride - Nasal DecongestantCold, Flu & Hay Fever Multi-Symptom Relief
■ Minor Aches & Pain, Sore Throat
■ Headache, Fever
■ Nasal Congestion, Sinus Pressure/Drainage
■ Sneezing, Runny Nose
■ Itchy, Watery Eyes24 Tablets
In Individually Sealed Strip
Aspirin Free

-
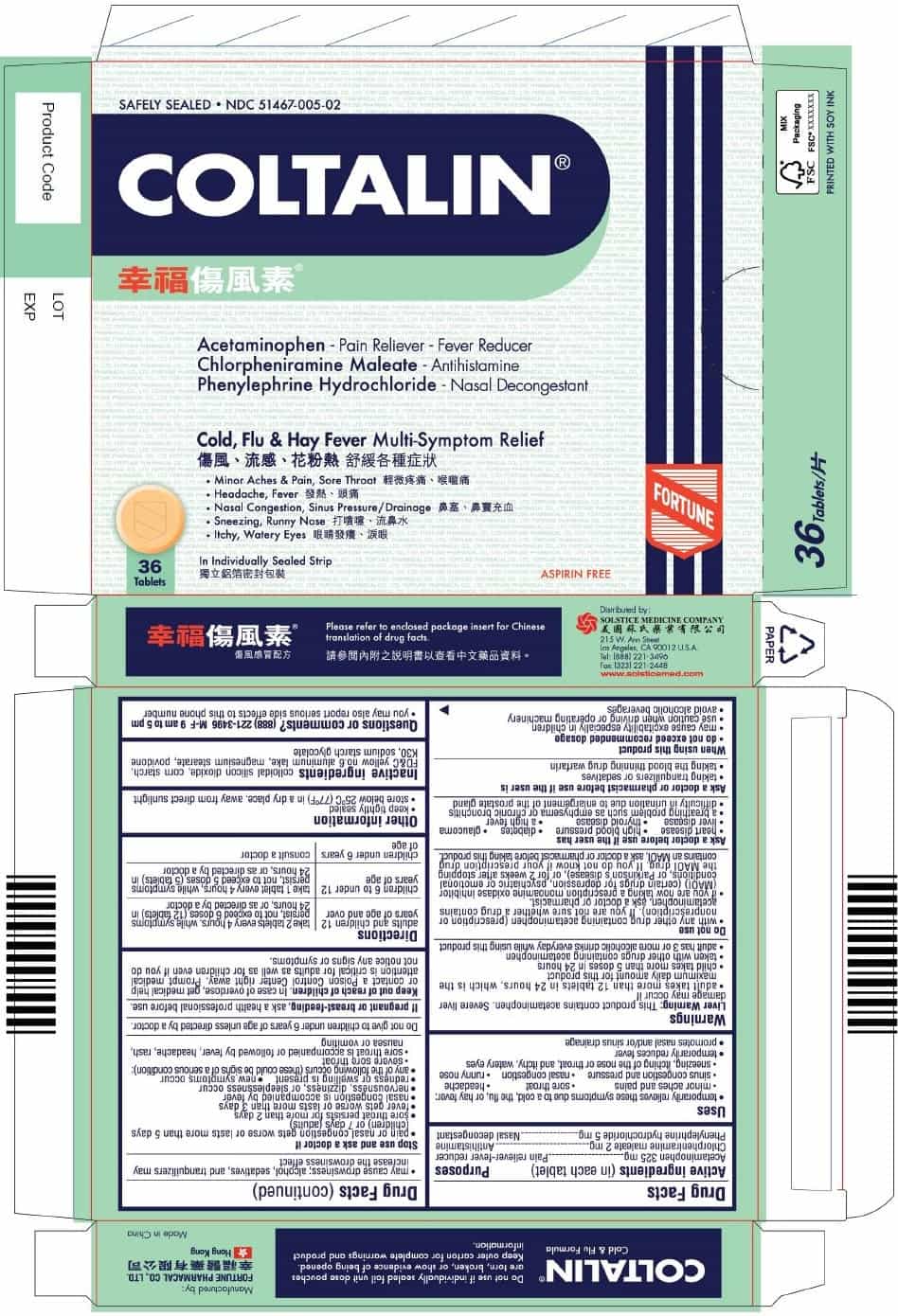
PRINCIPAL DISPLAY PANEL
COLTALIN
Safely Sealed
NDC 51467-005-02
Acetaminophen - Pain Reliever-Fever Reducer
Chlorpheniramine maleate - Antihistamine
Phenylephrine hydrochloride - Nasal DecongestantCold, Flu & Hay Fever Multi-Symptom Relief
■ Minor Aches & Pain, Sore Throat
■ Headache, Fever
■ Nasal Congestion, Sinus Pressure/Drainage
■ Sneezing, Runny Nose
■ Itchy, Watery Eyes36 Tablets
In Individually Sealed Strip
Aspirin Free
-
INGREDIENTS AND APPEARANCE
COLTALIN
acetaminophen, chlorpheniramine maleate, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51467-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color orange Score no score Shape ROUND Size 12mm Flavor Imprint Code COLTALIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51467-005-01 2 in 1 BOX 03/01/2004 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:51467-005-02 3 in 1 BOX 04/26/2016 2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 03/01/2004 Labeler - FORTUNE PHARMACAL COMPANY LIMITED (686280561) Registrant - FORTUNE PHARMACAL COMPANY LIMITED (686280561) Establishment Name Address ID/FEI Business Operations FORTUNE PHARMACAL COMPANY LIMITED 686280561 manufacture(51467-005)