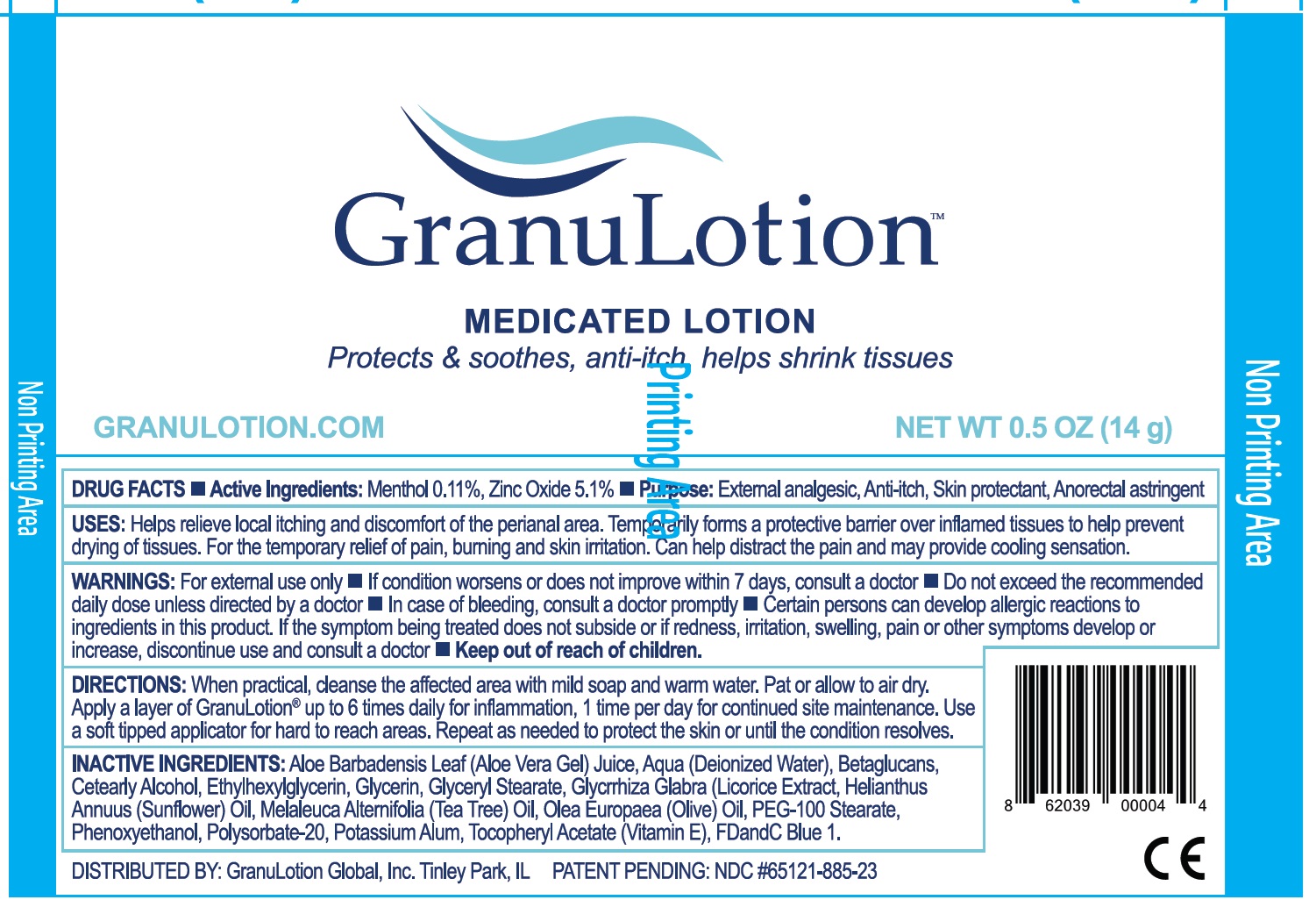
Label: GRANULOTION MEDICATED- menthol, zinc oxide lotion
- NDC Code(s): 82897-544-00
- Packager: GL HEALTH, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredients:
- USES:
-
WARNINGS:
For external use only
- If condition worsens or does not improve within 7 days, consult a doctor
- Do not exceed the recommended daily dose unless directed by a doctor
- In case of bleeding, consult a doctor promptly
- Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase, discontinue use and consult a doctor
-
DIRECTIONS:
When practical, cleanse the affected area with mild soap and warm water. Pat or allow to air dry. Apply a layer of GranuLotion® up to 6 times daily for inflammation, 1 time per day for continued site maintenance. Use a soft tipped applicator for hard to reach areas. Repeat as needed to protect the skin or until the condition resolves.
-
INACTIVE INGREDIENTS:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Betaglucans, Cetearyl Alcohol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Glycrrhiza Glabra (Licorice Extract, Helianthus Annuus (Sunflower) Oil, Melaleuca Alternifolia (Tea Tree) Oil, Olea Europaea (Olive) Oil, PEG-100 Stearate, Phenoxyethanol, Polysorbate-20, Potassium Alum, Tocopheryl Acetate (Vitamin E), FDandC Blue 1.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
GRANULOTION MEDICATED
menthol, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82897-544 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.1 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 51 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LICORICE (UNII: 61ZBX54883) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) TEA TREE OIL (UNII: VIF565UC2G) OLIVE OIL (UNII: 6UYK2W1W1E) PEG-100 MONOSTEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM ALUM (UNII: 1L24V9R23S) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82897-544-00 14 g in 1 TUBE; Type 0: Not a Combination Product 10/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 10/14/2024 Labeler - GL HEALTH, INC. (086010932)