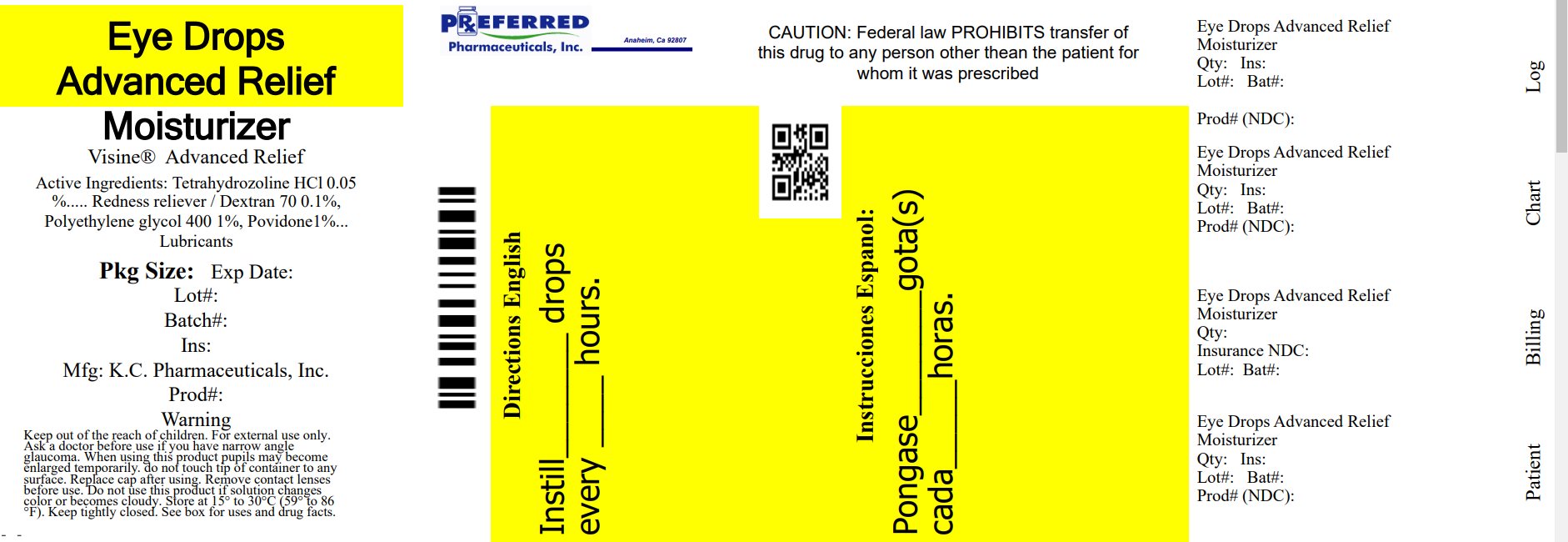
Label: GOODSENSE EYE DROPS ADVANCED RELIEF MOISTURIZER- dextran, polyethylene glycol 400, povidone, tetrahydrozoline hcl soluti...view full title
- NDC Code(s): 68788-8635-1
- Packager: Preferred PHarmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 50804-130
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive Ingredients - Dextran 70.....0.1% Polyethylene glycol 400.....1% Povidone.....1% tetrahydrozoline HCl.....0.05%
-
PURPOSEPurposes - Dextran 70.....Lubricant - Polyethylene glycol 400.....Lubricant - Povidone.....Lubricant - Tetrahydrozoline HCl .....Redness reliever
-
INDICATIONS & USAGEUses - • relieves redness of the eye due to minor eye irritations - • as a lubricant to prevent further irritation or to relieve dryness of the eye
-
WARNINGSWarnings - For external use only - Ask a doctor before use if you have narrow angle glaucoma Stop use and ask a doctor if you experience - • eye pain - • changes in vision - • continued ...
-
DOSAGE & ADMINISTRATIONDirections - Instill 1 to 2 drops in the affected eye(s) up to 4 times daily. Relabeled By: Preferred Pharmaceuticals Inc. NDC 68788-8635-1
-
INACTIVE INGREDIENTInactive ingredients - benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, and sodium chloride
-
PRINCIPAL DISPLAY PANEL
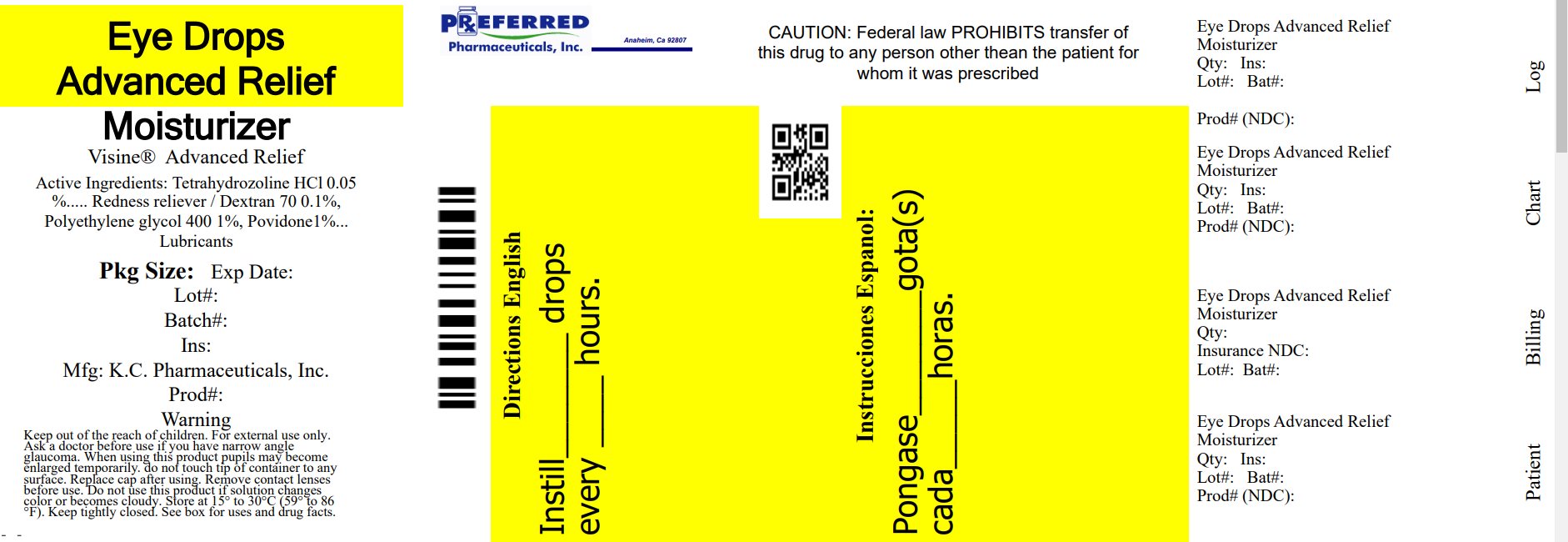
-
INGREDIENTS AND APPEARANCEProduct Information