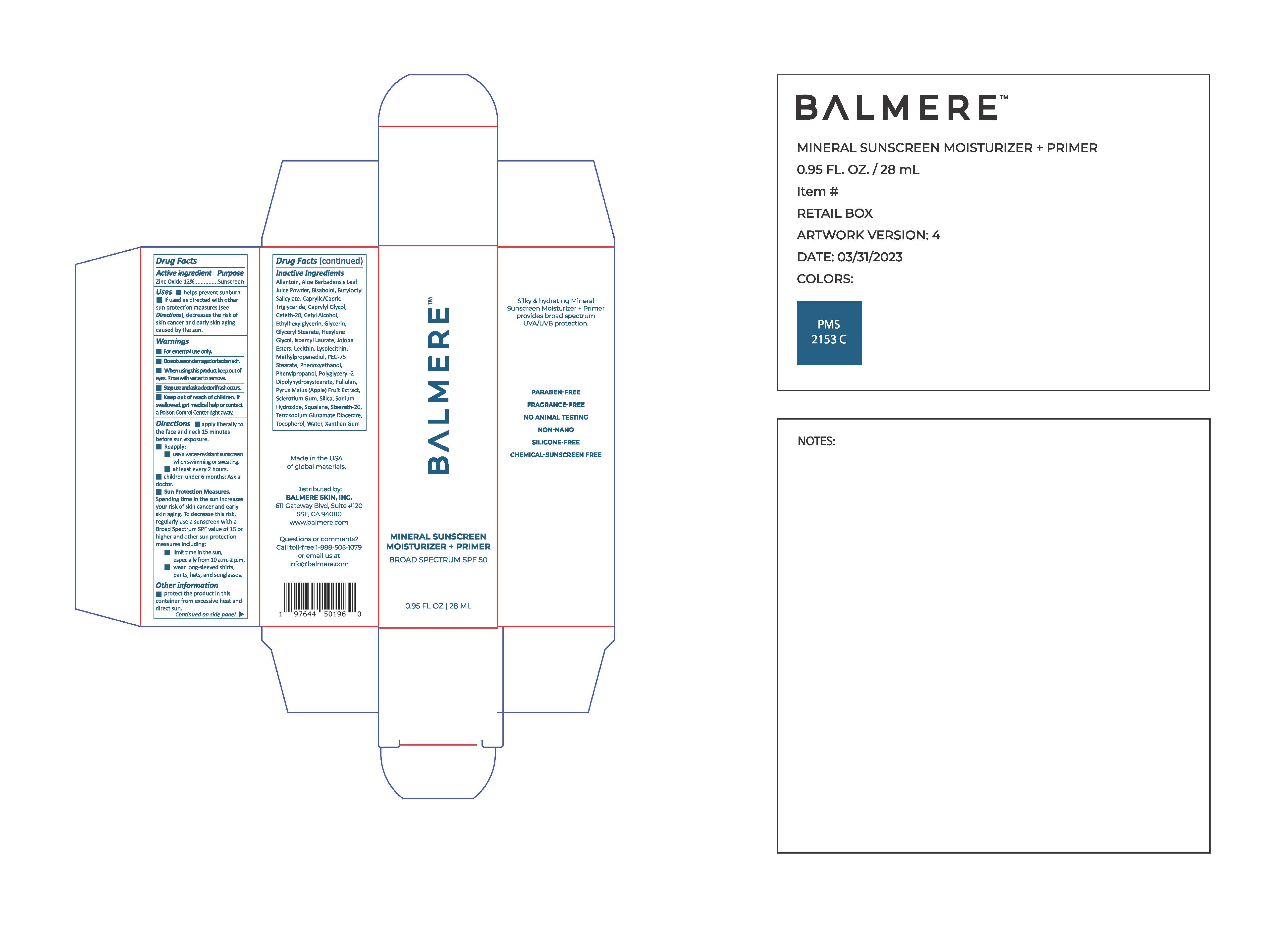
Label: BALMERE MINERAL SUNSCREEN MOISTURIZER PRIMER- zinc oxide lotion
- NDC Code(s): 83241-1039-1, 83241-1039-2
- Packager: Balmere
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
- Balmere Mineral Sunscreen Moisturizer + Primer
-
INGREDIENTS AND APPEARANCE
BALMERE MINERAL SUNSCREEN MOISTURIZER PRIMER
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83241-1039 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 132 mg in 1 mL Inactive Ingredients Ingredient Name Strength LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENYLPROPANOL (UNII: 0F897O3O4M) XANTHAN GUM (UNII: TTV12P4NEE) ISOAMYL LAURATE (UNII: M1SLX00M3M) HEXYLENE GLYCOL (UNII: KEH0A3F75J) TOCOPHEROL (UNII: R0ZB2556P8) CETYL ALCOHOL (UNII: 936JST6JCN) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETETH-20 (UNII: I835H2IHHX) PULLULAN (UNII: 8ZQ0AYU1TT) SQUALANE (UNII: GW89575KF9) STEARETH-20 (UNII: L0Q8IK9E08) CYCLODEXTRINS (UNII: 7E6SK9QDT8) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83241-1039-2 1 in 1 CARTON 05/20/2023 1 NDC:83241-1039-1 28 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/20/2023 Labeler - Balmere (118839840)