Label: GABLOFEN- baclofen injection injection, solution
-
NDC Code(s):
66794-151-01,
66794-155-01,
66794-155-02,
66794-156-01, view more66794-156-02, 66794-157-01, 66794-157-02
- Packager: Piramal Critical Care Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GABLOFEN - ® safely and effectively. See full prescribing information for GABLOFEN. GABLOFEN (baclofen injection), for intrathecal ...These highlights do not include all the information needed to use GABLOFEN ® safely and effectively. See full prescribing information for GABLOFEN.
GABLOFEN (baclofen injection), for intrathecal use
Initial U.S. Approval: 1992WARNING: DO NOT DISCONTINUE ABRUPTLY
See full prescribing information for complete boxed warning
Abrupt discontinuation of intrathecal baclofen, regardless of the cause, has resulted in sequelae that include high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure and death.
Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal. Special attention should be given to patients at apparent risk (e.g., spinal cord injuries at T-6 or above, communication difficulties, history of withdrawal symptoms from oral or intrathecal baclofen). Consult the technical manual of the implantable infusion system for additional post-implant clinician and patient information. ( 5.4)INDICATIONS AND USAGE
· GABLOFEN is a gamma-aminobutyric acid (GABA) ergic agonist indicated for use in the management of severe spasticity of cerebral or spinal origin in adult and pediatric patients age 4 years and above. ( 1)
· GABLOFEN should be reserved for patients unresponsive to oral baclofen therapy, or those who experience intolerable central nervous system side effects at effective doses. ( 1)
· Patients should first respond to a screening dose of intrathecal baclofen prior to consideration for long term infusion via an implantable pump. ( 1)
· Spasticity due to traumatic brain injury: wait at least one year after injury before considering GABLOFEN therapy. ( 1)
DOSAGE AND ADMINISTRATION
· GABLOFEN is intended for use by the intrathecal route in single bolus test doses (via spinal catheter or lumbar puncture) and, for chronic use in the Medtronic SynchroMed ® II Programmable Pump or other pumps labeled for intrathecal administration of GABLOFEN; Refer to the pump manufacturer's manual and follow the specific instructions and precautions for programming the pump and/or refilling the reservoir. ( 2.1)
· Screening: Patients who do not respond to a 100 mcg intrathecal bolus should not be considered for an implanted pump for chronic infusion. ( 2.2)
· Dose Titration: Spasticity may be necessary to sustain upright posture and balance in locomotion or may be useful to obtain optimal function and care. ( 2.5)
· Maintenance Therapy: Titrate patients individually; Lowest dose with an optimal response should be used, generally 300 mcg/day to 800 mcg/day for spasticity of spinal cord origin and 90 mcg/day to 700 mcg/day for spasticity of cerebral origin; Titrate GABLOFEN to maintain some degree of muscle tone and allow occasional spasms. ( 2.6)
DOSAGE FORMS AND STRENGTHS
Injection: 50 mcg/mL, 500 mcg/mL, 1,000 mcg/mL, 2,000 mcg/mL ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
· Do not directly inject GABLOFEN into the pump catheter access port, as this may cause a life-threatening overdose. (5.1) (5)
· Potential for contamination due to non-sterile external surface of prefilled syringe. (5.2) (5)
· Potentially life-threatening CNS depression, cardiovascular collapse, and/or respiratory failure; Resuscitative equipment and trained staff must be available during screening, dose titration, and refills (5.3) (5)
· Overdose may cause drowsiness, lightheadedness, dizziness, somnolence, respiratory depression, seizures, rostral progression of hypotonia and loss of consciousness progressing to coma. (5.4) (5)
· Possible exacerbation of psychotic disorders, schizophrenia or confusional states (5.6) (5)
ADVERSE REACTIONS
· The most common adverse reactions in patients with spasticity of spinal origin were somnolence, dizziness, nausea, hypotension, headache, convulsions and hypotonia. (6.1) (6)
· The most common adverse reactions in patients with spasticity of cerebral origin were agitation, constipation, somnolence, leukocytosis, chills, urinary retention and hypotonia. (6.2) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Piramal Critical Care, Inc. at 1-888-822-8431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
BOXED WARNING
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: DO NOT DISCONTINUE ABRUPTLY
Close
Abrupt discontinuation of intrathecal baclofen, regardless of the cause, has resulted in sequelae that include high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity, that in rare cases has advanced to rhabdomyolysis, multiple organ-system failure and death. Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal. Special attention should be given to patients at apparent risk (e.g., spinal cord injuries at T-6 or above, communication difficulties, history of withdrawal symptoms from oral or intrathecal baclofen). Consult the technical manual of the implantable infusion system for additional post-implant clinician and patient information [ see Warnings and Precautions (5.4)]. -
1 INDICATIONS AND USAGEGABLOFEN is indicated for use in the management of severe spasticity in adult and pediatric patients age 4 years and above. Patients should first respond to a screening dose of intrathecal ...
GABLOFEN is indicated for use in the management of severe spasticity in adult and pediatric patients age 4 years and above. Patients should first respond to a screening dose of intrathecal baclofen prior to consideration for long term infusion via an implantable pump. For spasticity of spinal cord origin, chronic infusion of GABLOFEN via an implantable pump should be reserved for patients unresponsive to oral baclofen therapy, or those who experience intolerable CNS side effects at effective doses. Patients with spasticity due to traumatic brain injury should wait at least one year after the injury before consideration of long term intrathecal baclofen therapy. GABLOFEN is intended for use by the intrathecal route in single bolus test doses (via spinal catheter or lumbar puncture) and, for chronic use, only with the Medtronic SynchroMed ® II Programmable Pump or other pumps labeled for intrathecal administration of GABLOFEN [see Clinical Studies (14)].
Prior to implantation of a device for chronic intrathecal infusion of GABLOFEN, patients must show a response to GABLOFEN in a screening trial [see Dosage and Administration (2.2)] .
Close -
2 DOSAGE AND ADMINISTRATION2.1 Use Only in Medtronic SynchroMed - ® II Programmable Pump (or other pumps labeled for intrathecal administration of GABLOFEN) GABLOFEN is approved only for ...
2.1 Use Only in Medtronic SynchroMed ® II Programmable Pump (or other pumps labeled for intrathecal administration of GABLOFEN)
GABLOFEN is approved only for use with the Medtronic SynchroMed ® II Programmable Pump or other pumps labeled for intrathecal administration of GABLOFEN. Refer to the manufacturer’s manual for specific instructions and precautions for programming the pump and/or refilling the reservoir. It is important to select the appropriate refill kit for the pump used to administer GABLOFEN. GABLOFEN is not to be compounded with other medications.
2.2 Screening Phase
Prior to pump implantation and initiation of chronic infusion of GABLOFEN, patients must demonstrate a positive clinical response to a GABLOFEN bolus dose administered intrathecally in a screening trial. The screening trial employs GABLOFEN at a concentration of 50 mcg/mL. A 1 mL syringe (50 mcg/mL) is available for use in the screening trial. The screening procedure is as follows. An initial bolus containing 50 micrograms in a volume of 1 milliliter is administered into the intrathecal space by barbotage over a period of not less than one minute. The patient is observed over the ensuing 4 to 8 hours. A positive response consists of a significant decrease in muscle tone and/or frequency and/or severity of spasms. If the initial response is less than desired, a second bolus injection may be administered 24 hours after the first. The second screening bolus dose consists of 75 micrograms in 1.5 milliliters. Again, the patient should be observed for an interval of 4 to 8 hours. If the response is still inadequate, a final bolus screening dose of 100 micrograms in 2 milliliters may be administered 24 hours later.
Pediatric Patients
The starting screening dose for pediatric patients is the same as in adult patients, i.e., 50 mcg. However, for very small patients, a screening dose of 25 mcg may be tried first.
Patients who do not respond to a 100 mcg intrathecal bolus should not be considered candidates for an implanted pump for chronic infusion.
2.3 Preparation Information
Screening
Use the 1 mL screening syringe only (50 mcg/mL) for bolus injection into the subarachnoid space. For a 50 mcg bolus dose, use 1 mL of the screening syringe. Use 1.5 mL of 50 mcg/mL baclofen injection for a 75 mcg bolus dose. For the maximum screening dose of 100 mcg, use 2 mL of 50 mcg/mL baclofen injection (2 screening syringes).
Maintenance
The specific concentration that should be used depends upon the total daily dose required as well as the delivery rate of the pump. For patients who require concentrations other than 500 mcg/mL, 1,000 mcg/mL, or 2,000 mcg/mL, GABLOFEN must be diluted with sterile preservative free Sodium Chloride for Injection, USP.
2.4 Administration Information
Parenteral drug products should be inspected for particulate matter and discoloration prior to administration, whenever solution and container permit.
The external surface of GABLOFEN prefilled syringes (all strengths, including the 50 mcg/mL strength) are non-sterile. The use of GABLOFEN prefilled syringe in an aseptic setting (i.e., operating room) to fill sterile intrathecal pumps prior to implantation in patients is not recommended. For outpatient use, modify aseptic procedures to avoid contamination of sterile surfaces through contact with the non-sterile exterior of the GABLOFEN prefilled syringe when filling the pump reservoir [see Warnings and Precautions ( 5.2)].
Delivery Regimen
GABLOFEN is most often administered in a continuous infusion mode immediately following implant. For those patients implanted with programmable pumps who have achieved relatively satisfactory control on continuous infusion, further benefit may be attained using more complex schedules of GABLOFEN delivery. For example, patients who have increased spasms at night may require a 20% increase in their hourly infusion rate. Changes in flow rate should be programmed to start two hours before the time of desired clinical effect.
2.5 Dose Titration
Post-Implant Dose Titration Period
To determine the initial total daily dose of GABLOFEN following implant, the screening dose that gave a positive effect should be doubled and administered over a 24-hour period, unless the efficacy of the bolus dose was maintained for more than 8 hours, in which case the starting daily dose should be the screening dose delivered over a
24-hour period. No dose increases should be given in the first 24 hours (i.e., until the steady state is achieved). In most patients, it will be necessary to increase the dose gradually over time to maintain effectiveness; a sudden requirement for substantial dose escalation typically indicates a catheter complication (i.e., catheter kink or dislodgement).Adult Patients with Spasticity of Spinal Cord Origin
After the first 24 hours, for adult patients, the daily dosage should be increased slowly by 10% to 30% increments and only once every 24 hours, until the desired clinical effect is achieved.
Adult Patients with Spasticity of Cerebral Origin
After the first 24 hours, the daily dose should be increased slowly by 5% to 15% only once every 24 hours, until the desired clinical effect is achieved.
Pediatric Patients
After the first 24 hours, the daily dose should be increased slowly by 5% to 15% only once every 24 hours, until the desired clinical effect is achieved. If there is not a substantive clinical response to increases in the daily dose, check for proper pump function and catheter patency. Patients must be monitored closely in a fully equipped and staffed environment during the screening phase and dose-titration period immediately following implant. Resuscitative equipment should be immediately available for use in case of life-threatening or intolerable side effects.
Additional Considerations Pertaining to Dosage Adjustment
Careful dose titration of GABLOFEN is needed when spasticity is necessary to sustain upright posture and balance in locomotion or whenever spasticity is used to obtain optimal function and care. It may be important to titrate the dose to maintain some degree of muscle tone and allow occasional spasms to: 1) help support circulatory function, 2) possibly prevent the formation of deep vein thrombosis, 3) optimize activities of daily living and ease of care.
Except in overdose related emergencies, the dose of GABLOFEN should ordinarily be reduced slowly if the drug is discontinued for any reason.
An attempt should be made to discontinue concomitant oral antispasticity medication to avoid possible overdose or adverse drug interactions, either prior to screening or following implant and initiation of chronic GABLOFEN infusion. Reduction and discontinuation of oral anti-spasmotics should be done slowly and with careful monitoring by the physician. Abrupt reduction or discontinuation of concomitant antispastics should be avoided.
2.6 Maintenance Therapy
Spasticity of Spinal Cord Origin Patients
The clinical goal is to maintain muscle tone as close to normal as possible, and to minimize the frequency and severity of spasms to the extent possible, without inducing intolerable side effects. Very often, the maintenance dose needs to be adjusted during the first few months of therapy while patients adjust to changes in lifestyle due to the alleviation of spasticity. During periodic refills of the pump, the daily dose may be increased by 10% to 40%, but no more than 40%, to maintain adequate symptom control. The daily dose may be reduced by 10% to 20% if patients experience side effects. Most patients require gradual increases in dose over time to maintain optimal response during chronic therapy. A sudden large requirement for dose escalation suggests a catheter complication (i.e., catheter kink or dislodgement).
Maintenance dosage for long term continuous infusion of intrathecal baclofen has ranged from 12 mcg/day to 2,003 mcg/day, with most patients adequately maintained on 300 micrograms to 800 micrograms per day. There is limited experience with daily doses greater than 1,000 mcg/day. Determination of the optimal GABLOFEN dose requires individual titration. The lowest dose with an optimal response should be used.
Spasticity of Cerebral Origin Patients
The clinical goal is to maintain muscle tone as close to normal as possible and to minimize the frequency and severity of spasms to the extent possible, without inducing intolerable side effects, or to titrate the dose to the desired degree of muscle tone for optimal functions. Very often the maintenance dose needs to be adjusted during the first few months of therapy while patients adjust to changes in lifestyle due to the alleviation of spasticity.
During periodic refills of the pump, the daily dose may be increased by 5% to 20%, but no more than 20%, to maintain adequate symptom control. The daily dose may be reduced by 10% to 20% if patients experience side effects. Many patients require gradual increases in dose over time to maintain optimal response during chronic therapy. A sudden large requirement for dose escalation suggests a catheter complication (i.e., catheter kink or dislodgement).
Maintenance dosage for long term continuous infusion of intrathecal baclofen has ranged from 22 mcg/day to 1,400 mcg/day, with most patients adequately maintained on 90 micrograms to 703 micrograms per day. In clinical trials, only 3 of 150 patients required daily doses greater than 1,000 mcg/day.
Pediatric Patients
Use same dosing recommendations for patients with spasticity of cerebral origin. Pediatric patients under 12 years seemed to require a lower daily dose in clinical trials. Average daily dose for patients under 12 years was 274 mcg/day, with a range of 24 mcg/day to 1,199 mcg/day. Dosage requirement for pediatric patients over 12 years does not seem to be different from that of adult patients. Determination of the optimal GABLOFEN dose requires individual titration. The lowest dose with an optimal response should be used.
Potential Need for Dose Adjustments in Chronic Use
During long term treatment, approximately 5% (28/627) of patients become refractory to increasing doses. There is not sufficient experience to make firm recommendations for tolerance treatment; however, this “tolerance” has been treated on occasion, in hospital, by a “drug holiday” consisting of the gradual reduction of intrathecal baclofen over a 2 to 4 week period and switching to alternative methods of spasticity management. After the “drug holiday,” intrathecal baclofen may be restarted at the initial continuous infusion dose.
Close -
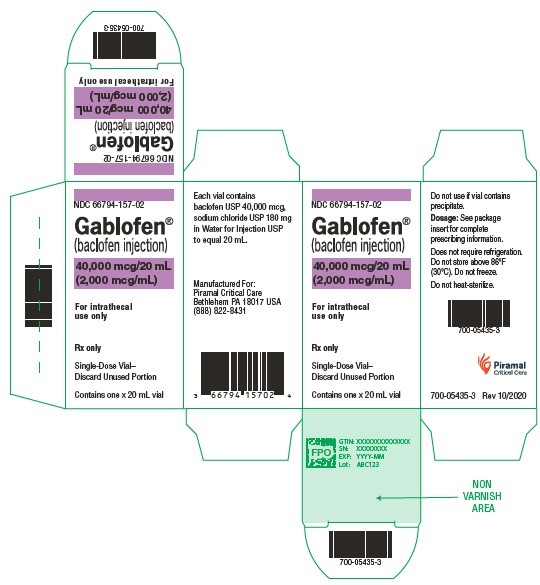
3 DOSAGE FORMS AND STRENGTHSGABLOFEN (baclofen injection) is available, for intrathecal use only, in: Single-dose syringe of 1 mL containing 50 mcg (50 mcg/mL) Single-dose syringes and vials of 10,000 mcg per 20 mL (500 ...
GABLOFEN (baclofen injection) is available, for intrathecal use only, in:
- Single-dose syringe of 1 mL containing 50 mcg (50 mcg/mL)
- Single-dose syringes and vials of 10,000 mcg per 20 mL (500 mcg/mL)
- Single-dose syringes and vials of 20,000 mcg per 20 mL (1,000 mcg/mL)
- Single-dose syringes and vials of 40,000 mcg per 20 mL (2,000 mcg/mL)
-
4 CONTRAINDICATIONSGABLOFEN is contraindicated in patients with a hypersensitivity to baclofen. Do not use GABLOFEN for intravenous, intramuscular, subcutaneous or epidural administration.
GABLOFEN is contraindicated in patients with a hypersensitivity to baclofen. Do not use GABLOFEN for intravenous, intramuscular, subcutaneous or epidural administration.
Close -
5 WARNINGS AND PRECAUTIONS5.1 Risk of Life-Threatening Overdose During Pump Refills - Use extreme caution when filling the Medtronic SynchroMed - ® II Programmable Pump which is equipped with an injection ...
5.1 Risk of Life-Threatening Overdose During Pump Refills
Use extreme caution when filling the Medtronic SynchroMed ® II Programmable Pump which is equipped with an injection port that allows direct access to the intrathecal catheter. Direct injection into the catheter through the catheter access port may cause a life-threatening overdose.
Reservoir refilling must be performed by fully trained and qualified personnel following the directions provided by the pump manufacturer. Carefully calculate refill intervals to prevent depletion of the reservoir, as this would result in the return of severe spasticity and possibly symptoms of withdrawal.
Strict aseptic technique in filling is required to avoid bacterial contamination and serious infection. A period of observation appropriate to the clinical situation should follow each refill or manipulation of the drug reservoir.
5.2 Potential for Contamination due to Non-sterile External Surface of Prefilled Syringe
Although the drug solution and pathway in the GABLOFEN prefilled syringes are sterile, the external surface of the prefilled syringes (all strengths, including the 50 mcg/mL strength) are non-sterile. This has the potential to lead to contamination and consequent adverse reactions. The use of GABLOFEN prefilled syringe in an aseptic setting (e.g., operating room) to fill sterile intrathecal pumps prior to implantation in patients is not recommended, unless the external surface of the prefilled syringe is treated to ensure sterility. GABLOFEN supplied in vials may be used with conventional aseptic technique to fill intrathecal pumps prior to implantation. Procedures should also be put in place while refilling implantable intrathecal pumps in an outpatient setting to avoid contamination of sterile surfaces through contact with the non-sterile exterior of the GABLOFEN prefilled syringe.
5.3 Prescriber, Caregiver and Patient Training and Screening Procedure/Post-Implantation Environment
GABLOFEN is for use in single bolus intrathecal injections (via a catheter placed in the lumbar intrathecal space or injection by lumbar puncture) and in the implantable Medtronic SynchroMed® II Programmable Pump or other pumps labeled for intrathecal administration of GABLOFEN. Because of the possibility of potentially life-threatening CNS depression, cardiovascular collapse, and/or respiratory failure, physicians must be adequately trained and educated in chronic intrathecal infusion therapy.
The pump system should not be implanted until the patient's response to bolus GABLOFEN injection is adequately evaluated. Evaluation (consisting of a screening procedure) requires that GABLOFEN be administered into the intrathecal space via a catheter or lumbar puncture [see Dosage and Administration (2.2)]. Because of the risks associated with the screening procedure and the adjustment of dosage following pump implantation, these phases must be conducted in a medically supervised and adequately equipped environment following the instructions outlined in the Dosage and Administration section [see Dosage and Administration (2.2 and 2.5)] .
Resuscitative equipment should be available.
Following surgical implantation of the pump, particularly during the initial phases of pump use, the patient should be monitored closely until it is certain that the patient's response to the infusion is acceptable and reasonably stable.
On each occasion that the dosing rate of the pump and/or the concentration of GABLOFEN in the reservoir is adjusted, close medical monitoring is required until it is certain that the patient's response to the infusion is acceptable and reasonably stable.
It is mandatory that the patient, all patient caregivers, and the physicians responsible for the patient receive adequate information regarding the risks of this mode of treatment. All medical personnel and caregivers should be instructed in 1) the signs and symptoms of overdose, 2) procedures to be followed in the event of overdose and 3) proper home care of the pump and insertion site.
5.4 Overdose
Signs of overdose may appear suddenly or insidiously. Acute massive overdose may present as coma. Less sudden and/or less severe forms of overdose may present with signs of drowsiness, lightheadedness, dizziness, somnolence, respiratory depression, seizures, rostral progression of hypotonia and loss of consciousness progressing to coma. Should overdose appear likely, the patient should be taken immediately to a hospital for assessment and emptying of the pump reservoir. In cases reported to date, overdose has generally been related to pump malfunction or dosing error [see Overdosage ( 10)]
Extreme caution must be used when filling the implantable pump.
Medtronic SynchroMed ® II Programmable Pump should only be refilled through the reservoir refill septum. The Medtronic SynchroMed ® II Programmable Pump is also equipped with a catheter access port that allows direct access to the intrathecal catheter. Direct injection into this catheter access port may cause a life-threatening overdose.
5.5 Withdrawal
Abrupt withdrawal of intrathecal baclofen, regardless of the cause, has resulted in sequelae that included high fever, altered mental status, exaggerated rebound spasticity and muscle rigidity that in rare cases progressed to rhabdomyolysis, multiple organ-system failure, and death. In the first 9 years of post-marketing experience, 27 cases of withdrawal temporally related to the cessation of baclofen therapy were reported; six patients died. In most cases, symptoms of withdrawal appeared within hours to a few days following interruption of baclofen therapy. Common reasons for abrupt interruption of intrathecal baclofen therapy included malfunction of the catheter (especially disconnection), low volume in the pump reservoir, and end of pump battery life; human error may have played a causal or contributing role in some cases. Cases of intrathecal mass at the tip of the implanted catheter leading to withdrawal symptoms have also been reported, most of them involving pharmacy compounded analgesic admixtures [see Warnings and Precautions (5.10)].
Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal.
All patients receiving intrathecal baclofen therapy are potentially at risk for withdrawal. Early symptoms of baclofen withdrawal may include return of baseline spasticity, pruritus, hypotension, and paresthesias. Some clinical characteristics of the advanced intrathecal baclofen withdrawal syndrome may resemble autonomic dysreflexia, infection (sepsis), malignant hyperthermia, neuroleptic-malignant syndrome, or other conditions associated with a hypermetabolic state or widespread rhabdomyolysis.
Rapid, accurate diagnosis and treatment in an emergency-room or intensive-care setting are important in order to prevent the potentially life-threatening central nervous system and systemic effects of intrathecal baclofen withdrawal. The suggested treatment for intrathecal baclofen withdrawal is the restoration of intrathecal baclofen at or near the same dosage as before therapy was interrupted. However, if restoration of intrathecal delivery is delayed, treatment with GABA-ergic agonist drugs such as oral or enteral baclofen, or oral, enteral, or intravenous benzodiazepines may prevent potentially fatal sequelae. Oral or enteral baclofen alone should not be relied upon to halt the progression of intrathecal baclofen withdrawal.
Seizures have been reported during overdose and with withdrawal from intrathecal baclofen as well as in patients maintained on therapeutic doses of intrathecal baclofen.
5.6 Possible Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States
Patients suffering from psychotic disorders, schizophrenia, or confusional states should be treated cautiously with GABLOFEN and kept under careful surveillance, because exacerbations of these conditions have been observed with oral administration.
5.7 Fatalities
Spasticity of Spinal Cord Origin
There were 16 deaths reported among the 576 U.S. patients treated with intrathecal baclofen in pre- and post-marketing studies evaluated as of December 1992. Because these patients were treated under uncontrolled clinical settings, it is impossible to determine definitively what role, if any, intrathecal baclofen played in their deaths. As a group, the patients who died were relatively young (mean age was 47 with a range from 25 to 63), but the majority suffered from severe spasticity of many years duration, were nonambulatory, had various medical complications such as pneumonia, urinary tract infections, and decubiti, and/or had received multiple concomitant medications. A case-by-case review of the clinical course of the 16 patients who died failed to reveal any unique signs, symptoms, or laboratory results that would suggest that treatment with intrathecal baclofen caused their deaths. Two patients, however, did suffer sudden and unexpected death within 2 weeks of pump implantation and one patient died unexpectedly after screening.
One patient, a 44 year-old male with Multiple Sclerosis, died in hospital on the second day following pump implantation. An autopsy demonstrated severe fibrosis of the coronary conduction system. A second patient, a 52 year-old woman with MS and a history of an inferior wall myocardial infarction, was found dead in bed 12 days after pump implantation, 2 hours after having had documented normal vital signs. An autopsy revealed pulmonary congestion and bilateral pleural effusions. It is impossible to determine whether intrathecal baclofen contributed to these deaths. The third patient underwent three baclofen screening trials. His medical history included spinal cord injury, aspiration pneumonia, septic shock, disseminated intravascular coagulopathy, severe metabolic acidosis, hepatic toxicity, and status epilepticus. Twelve days after screening (he was not implanted), he again experienced status epilepticus with subsequent significant neurological deterioration. Based upon prior instruction, extraordinary resuscitative measures were not pursued and the patient died.
Spasticity of Cerebral Origin
There were three deaths occurring among the 211 patients treated with intrathecal baclofen in pre-marketing studies as of March 1996. These deaths were not attributed to the therapy.
5.8 Use with Caution in Patients with a History of Autonomic Dysreflexia
GABLOFEN should be used with caution in patients with a history of autonomic dysreflexia. The presence of nociceptive stimuli or abrupt withdrawal of GABLOFEN may cause an autonomic dysreflexic episode.
5.9 Infections
Patients should be infection-free prior to the screening trial with GABLOFEN because the presence of a systemic infection may interfere with an assessment of the patient's response to bolus GABLOFEN. Patients should be infection-free prior to implantation of the pump because the presence of infection may increase the risk of surgical complications. Moreover, a systemic infection may complicate dosing.
5.10 Drowsiness
Drowsiness has been reported in patients on intrathecal baclofen. Patients should be cautioned regarding the operation of automobiles or other dangerous machinery, and activities made hazardous by decreased alertness. Patients should also be cautioned that the central nervous system depressant effects of intrathecal baclofen may be additive to those of alcohol and other CNS depressants.
5.11 Intrathecal Mass Formation
Cases of intrathecal mass at the tip of the implanted catheter have been reported, most of them involving pharmacy compounded analgesic admixtures. The most frequent symptoms associated with intrathecal mass are: 1) decreased therapeutic response (worsening spasticity, return of spasticity when previously well controlled, withdrawal symptoms, poor response to escalating doses, or frequent or large dosage increases), 2) pain, 3) neurological deficit/dysfunction. Clinicians should monitor patients on intraspinal therapy carefully for any new neurological signs or symptoms. In patients with new neurological signs or symptoms suggestive of an intrathecal mass, consider a neurosurgical consultation, since many of the symptoms of inflammatory mass are not unlike the symptoms experienced by patients with severe spasticity from their disease. In some cases, performance of an imaging procedure may be appropriate to confirm or rule-out the diagnosis of an intrathecal mass.
5.12 Ovarian Cysts
A dose-related increase in incidence of ovarian cysts was observed in female rats treated chronically with oral baclofen. Ovarian cysts have been found by palpation in about 4% of the multiple sclerosis patients who were treated with oral baclofen for up to one year. In most cases these cysts disappeared spontaneously while patients continued to receive the drug. Ovarian cysts are estimated to occur spontaneously in approximately 1% to 5% of the normal female population.
Close -
6 ADVERSE REACTIONS6.1 Spasticity of Spinal Cord Origin - Most Common Adverse Reactions in Patients with Spasticity of Spinal Origin - In pre- and post-marketing clinical trials, the most common adverse ...
6.1 Spasticity of Spinal Cord Origin
Most Common Adverse Reactions in Patients with Spasticity of Spinal Origin
In pre- and post-marketing clinical trials, the most common adverse reactions associated with use of intrathecal baclofen which were not seen at an equivalent incidence among placebo-treated patients were: somnolence, dizziness, nausea, hypotension, headache, convulsions and hypotonia.Adverse Reactions Associated with Discontinuation of Treatment
8/474 patients with spasticity of spinal cord origin receiving long term infusion of intrathecal baclofen in pre- and post-marketing clinical studies in the U.S. discontinued treatment due to adverse reactions. These include: pump pocket infections (3), meningitis (2), wound dehiscence (1), gynecological fibroids (1) and pump overpressurization (1) with unknown, if any, sequela. Eleven patients who developed coma secondary to overdose had their treatment temporarily suspended, but all were subsequently re-started and were not, therefore, considered to be true discontinuations.Fatalities - [see Warnings and Precautions (5.6)].
Incidence in Controlled Trials
Experience with intrathecal baclofen obtained in parallel, placebo-controlled, randomized studies provides only a limited basis for estimating the incidence of adverse reactions because the studies were of very brief duration (up to three days of infusion) and involved only a total of 63 patients. The following events occurred among the 31 patients receiving intrathecal baclofen in two randomized, placebo-controlled trials: hypotension (2), dizziness (2), headache (2), dyspnea (1). No adverse reactions were reported among the 32 patients receiving placebo in these studies.
Events Observed during the Pre- and Post-marketing Evaluation of Intrathecal Baclofen
Adverse events associated with the use of intrathecal baclofen reflect experience gained with 576 patients followed prospectively in the United States. They received intrathecal baclofen for periods of one day (screening) (N=576) to over eight years (maintenance) (N=10). The usual screening bolus dose administered prior to pump implantation in these studies was typically 50 mcg. The maintenance dose ranged from 12 mcg to 2,003 mcg per day. Because of the open, uncontrolled nature of the experience, a causal linkage between events observed and the administration of intrathecal baclofen cannot be reliably assessed in many cases and many of the adverse reactions reported are known to occur in association with the underlying conditions being treated. Nonetheless, many of the more commonly reported reactions — hypotonia, somnolence, dizziness, paresthesia, nausea/vomiting and headache — appear clearly drug-related.
Adverse experiences reported during all U.S. studies (both controlled and uncontrolled) are shown in Table 1. Eight of 474 patients who received chronic infusion via implanted pumps had adverse experiences which led to a discontinuation of long term treatment in the pre- and post-marketing studies.
Table 1: Most Common (≥1%) Adverse Reactions in Patients with Spasticity of Spinal Origin in Prospectively Monitored Clinical TrialsAdverse Reactions Percent
N=576
ScreeningPercent
N=474
Titration†Percent
N=430
Maintenance‡Hypotonia 5.4 13.5 25.3 Somnolence 5.7 5.9 20.9 Dizziness 1.7 1.9 7.9 Paresthesia 2.4 2.1 6.7 Nausea and Vomiting 1.6 2.3 5.6 Headache 1.6 2.5 5.1 Constipation 0.2 1.5 5.1 Convulsion 0.5 1.3 4.7 Urinary Retention 0.7 1.7 1.9 Dry Mouth 0.2 0.4 3.3 Accidental Injury 0.0 0.2 3.5 Asthenia 0.7 1.3 1.4 Confusion 0.5 0.6 2.3 Death 0.2 0.4 3.0 Pain 0.0 0.6 3.0 Speech Disorder 0.0 0.2 3.5 Hypotension 1.0 0.2 1.9 Ambylopia 0.5 0.2 2.3 Diarrhea 0.0 0.8 2.3 Hypoventilation 0.2 0.8 2.1 Coma 0.0 1.5 0.9 Impotence 0.2 0.4 1.6 Peripheral Edema 0.0 0.0 2.3 Urinary Incontinence 0.0 0.8 1.4 Insomnia 0.0 0.4 1.6 Anxiety 0.2 0.4 0.9 Depression 0.0 0.0 1.6 Dypsnea 0.3 0.0 1.2 Fever 0.5 0.2 0.7 Pneumonia 0.2 0.2 1.2 Urinary Frequency 0.0 0.6 0.9 Urticaria 0.2 0.2 1.2 Anorexia 0.0 0.4 0.9 Diplopia 0.0 0.4 0.9 Dysautonomia 0.2 0.2 0.9 Hallucinations 0.3 0.4 0.5 Hypertension 0.2 0.6 0.5 * Following administration of test bolus † Two month period following implant
‡ Beyond two months following implant
N=Total number of patients entering each period
%=% of patients evaluatedIn addition to the more common (1% or more) adverse reactions reported in the prospectively followed 576 domestic patients in pre- and post-marketing studies, experience from an additional 194 patients exposed to intrathecal baclofen from foreign studies has been reported. The following adverse reactions, not described in the table, and arranged in decreasing order of frequency, and classified by body system, were reported:
Nervous System: Abnormal gait, thinking abnormal, tremor, amnesia, twitching, vasodilatation, cerebrovascular accident, nystagmus, personality disorder, psychotic depression, cerebral ischemia, emotional lability, euphoria, hypertonia, ileus, drug dependence, incoordination, paranoid reaction and ptosis.
Digestive System: Flatulence, dysphagia, dyspepsia and gastroenteritis.
Cardiovascular: Postural hypotension, bradycardia, palpitations, syncope, arrhythmia ventricular, deep thrombophlebitis, pallor and tachycardia.
Respiratory: Respiratory disorder, aspiration pneumonia, hyperventilation, pulmonary embolus and rhinitis.
Urogenital: Hematuria and kidney failure.
Skin and Appendages: Alopecia and sweating.
Metabolic and Nutritional Disorders: Weight loss, albuminuria, dehydration and hyperglycemia.
Special Senses: Abnormal vision, abnormality of accommodation, photophobia, taste loss and tinnitus.
Body as a Whole: Suicide, lack of drug effect, abdominal pain, hypothermia, neck rigidity, chest pain, chills, face edema, flu syndrome and overdose.
Hemic and Lymphatic System: Anemia.6.2 Spasticity of Cerebral Origin
Most Common Adverse Reactions
In pre-marketing clinical trials, the most common adverse reactions associated with use of intrathecal baclofen which were not seen at an equivalent incidence among placebo-treated patients included: agitation, constipation, somnolence, leukocytosis, chills, urinary retention and hypotonia.
Adverse Reactions Associated with Discontinuation of Treatment
Nine of 211 patients receiving intrathecal baclofen in pre-marketing clinical studies in the U.S. discontinued long-term infusion due to adverse reactions associated with intrathecal therapy.
The nine adverse reactions leading to discontinuation were: infection (3), CSF leaks (2), meningitis (2), drainage (1), and unmanageable trunk control (1).
Fatalities
Three deaths, none of which were attributed to intrathecal baclofen, were reported in patients in clinical trials involving patients with spasticity of cerebral origin. See Warnings on other deaths reported in spinal spasticity patients.
Incidence in Controlled Trials
Experience with intrathecal baclofen obtained in parallel, placebo-controlled, randomized studies provides only a limited basis for estimating the incidence of adverse reactions because the studies involved a total of 62 patients exposed to a single 50 mcg intrathecal bolus. The following adverse reactions occurred among the 62 patients receiving intrathecal baclofen in two randomized, placebo-controlled trials involving cerebral palsy and head injury patients, respectively: agitation, constipation, somnolence, leukocytosis, nausea, vomiting, nystagmus, chills, urinary retention, and hypotonia.
Events Observed during the Pre-marketing Evaluation of Intrathecal Baclofen
Adverse events associated with the use of intrathecal baclofen reflect experience gained with a total of 211 U.S. patients with spasticity of cerebral origin, of whom 112 were pediatric patients (under age 16 at enrollment). They received intrathecal baclofen for periods of one day (screening) (N=211) to 84 months (maintenance) (N=1). The usual screening bolus dose administered prior to pump implantation in these studies was 50 mcg to 75 mcg. The maintenance dose ranged from 22 mcg to 1,400 mcg per day. Doses used in this patient population for long-term infusion are generally lower than those required for patients with spasticity of spinal cord origin.
Because of the open, uncontrolled nature of the experience, a causal linkage between events observed and the administration of intrathecal baclofen cannot be reliably assessed in many cases. Nonetheless, many of the more commonly reported reactions — somnolence, dizziness, headache, nausea, hypotension, hypotonia and coma — appear clearly drug-related.
The most frequent (≥1%) adverse reactions reported during all clinical trials are shown in Table 2. Nine patients discontinued long term treatment due to adverse reactions.
Table 2: Most Common (≥1%) Adverse Reactions in Patients with Spasticity of Cerebral OriginAdverse Reactions Percent
N=211
Screening*Percent
N=153
Titration†Percent
N=150
Maintenance‡Hypotonia 2.4 14.4 34.7 Somnolence 7.6 10.5 18.7 Headache 6.6 7.8 10.7 Nausea and Vomiting 6.6 10.5 4.0 Vomiting 6.2 8.5 4.0 Urinary Retention 0.9 6.5 8.0 Convulsion 0.9 3.3 10.0 Dizziness 2.4 2.6 8.0 Nausea 1.4 3.3 7.3 Hypoventilation 1.4 1.3 4.0 Hypertonia 0.0 0.7 6.0 Paresthesia 1.9 0.7 3.3 Hypotension 1.9 0.7 2.0 Increased Salivation 0.0 2.6 2.7 Back Pain 0.9 0.7 2.0 Constipation 0.5 1.3 2.0 Pain 0.0 0.0 4.0 Pruritus 0.0 0.0 4.0 Diarrhea 0.5 0.7 2.0 Peripheral Edema 0.0 0.0 3.3 Thinking Abnormal 0.5 1.3 0.7 Agitation 0.5 0.0 1.3 Asthenia 0.0 0.0 2.0 Chills 0.5 0.0 1.3 Coma 0.5 0.0 1.3 Dry Mouth 0.5 0.0 1.3 Pneumonia 0.0 0.0 2.0 Speech Disorder 0.5 0.7 0.7 Tremor 0.5 0.0 1.3 Urinary Incontinence 0.0 0.0 2.0 Urination Impaired 0.0 0.0 2.0 * Following administration of test bolus
† Two month period following implant
Close
‡ Beyond two months following implant
N=Total number of patients entering each period. 211 patients received drug; (1 of 212) received placebo only
The more common (1% or more) adverse reactions reported in the prospectively followed 211 patients exposed to intrathecal baclofen have been reported. In the total cohort, the following adverse reactions, not described in Table 2, and arranged in decreasing order of frequency, and classified by body system, were reported:
Nervous System: Akathisia, ataxia, confusion, depression, opisthotonos, amnesia, anxiety, hallucinations, hysteria, insomnia, nystagmus, personality disorder, reflexes decreased, and vasodilitation.
Digestive System: Dysphagia, fecal incontinence, gastrointestinal hemorrhage and tongue disorder.
Cardiovascular: Bradycardia.
Respiratory: Apnea, dyspnea and hyperventilation.
Urogenital: Abnormal ejaculation, kidney calculus, oliguria and vaginitis.
Skin and Appendages: Rash, sweating, alopecia, contact dermatitis and skin ulcer.
Special Senses: Abnormality of accommodation.
Body as a Whole: Death, fever, abdominal pain, carcinoma, malaise and hypothermia.
Hemic and Lymphatic System: Leukocytosis and petechial rash. -
7 DRUG INTERACTIONSThere is inadequate systematic experience with the use of intrathecal baclofen in combination with other medications to predict specific drug-drug interactions. Interactions attributed to the ...
There is inadequate systematic experience with the use of intrathecal baclofen in combination with other medications to predict specific drug-drug interactions. Interactions attributed to the combined use of GABLOFEN and epidural morphine include hypotension and dyspnea.
Close -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of GABLOFEN in pregnant women. In animal studies, oral administration of baclofen ...
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of GABLOFEN in pregnant women.
In animal studies, oral administration of baclofen to pregnant rats produced an increase in fetal malformations (see Data). There are no animal data on developmental risk associated with baclofen administered via continuous intrathecal infusion.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Baclofen given orally to pregnant rats has been shown to increase the incidence of omphaloceles (ventral hernias) in fetuses at a dose associated with maternal toxicity. This abnormality was not seen in mice or rabbits.
8.2 Lactation
Risk Summary
There is insufficient information regarding levels of baclofen in milk of nursing mothers receiving GABLOFEN. There are no adequate data on the effects of GABLOFEN on the breastfed infant or on milk production. At recommended oral doses, baclofen is present in human milk and withdrawal symptoms can occur in breastfed infants when maternal administration of baclofen is stopped or when breastfeeding is stopped.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GABLOFEN and any potential adverse effects on the breastfed infant from GABLOFEN or from the underlying maternal condition.
Close8.4 Pediatric Use
Children should be of sufficient body mass to accommodate the implantable pump for chronic infusion. Please consult pump manufacturer's manual for specific recommendations.
Safety and effectiveness in pediatric patients below the age of 4 have not been established.
-
10 OVERDOSAGESpecial attention must be given to recognizing the signs and symptoms of overdosage, especially during the initial screening and dose-titration phase of treatment, but also during re-introduction ...
Special attention must be given to recognizing the signs and symptoms of overdosage, especially during the initial screening and dose-titration phase of treatment, but also during re-introduction of GABLOFEN after a period of interruption in therapy.
Symptoms of Intrathecal Baclofen Overdose
Drowsiness, lightheadedness, dizziness, somnolence, respiratory depression, seizures, rostral progression of hypotonia and loss of consciousness progressing to coma of up to 72 hours duration. In most cases reported, coma was reversible without sequelae after drug was discontinued. Symptoms of intrathecal baclofen overdose were reported in a sensitive adult patient after receiving a 25 mcg intrathecal bolus.
Treatment Suggestions for Overdose
There is no specific antidote for treating overdoses of GABLOFEN; however, the following steps should ordinarily be undertaken:
1) Residual intrathecal baclofen solution should be removed from the pump as soon as possible.
2) Patients with respiratory depression should be intubated if necessary, until the drug is eliminated.
Anecdotal reports suggest that intravenous physostigmine may reverse central side effects, notably drowsiness and respiratory depression. Caution in administering physostigmine is advised, however, because its use has been associated with the induction of seizures and bradycardia.
Physostigmine Doses for Adult Patients
Administer 2 mg of physostigmine intramuscularly or intravenously at a slow controlled rate of no more than 1 mg per minute. Dosage may be repeated if life-threatening signs, such as arrhythmia, convulsions or coma occur.
Physostigmine Doses for Pediatric Patients
Administer 0.02 mg/kg physostigmine intramuscularly or intravenously, do not give more than 0.5 mg per minute. The dosage may be repeated at 5 to 10 minute intervals until a therapeutic effect is obtained or a maximum dose of 2 mg is attained.
Physostigmine may not be effective in reversing large overdoses and patients may need to be maintained with respiratory support.
If lumbar puncture is not contraindicated, consideration should be given to withdrawing 30 to 40 mL of CSF to reduce CSF baclofen concentration.
Close -
11 DESCRIPTIONGABLOFEN (baclofen injection) is a muscle relaxant and antispastic. Baclofen's pharmacological class is a gamma-aminobutyric acid (GABA) ergic agonist. Baclofen's chemical name is ...
GABLOFEN (baclofen injection) is a muscle relaxant and antispastic. Baclofen's pharmacological class is a gamma-aminobutyric acid (GABA) ergic agonist. Baclofen's chemical name is 4-amino-3-(4-chlorophenyl) butanoic acid, and its structural formula is:
Baclofen
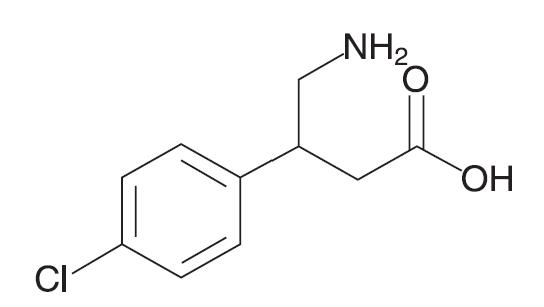
C 10H 12ClNO 2
Baclofen is a white to off-white powder, with a molecular weight of 213.66. It is slightly soluble in water, very slightly soluble in methanol and ethanol, practically insoluble in acetone and ether, soluble in 0.1N hydrochloric acid, 0.1N sodium hydroxide, and insoluble in chloroform.
GABLOFEN is a sterile, pyrogen-free, isotonic solution free of antioxidants, preservatives or other potentially neurotoxic additives indicated only for intrathecal administration. The drug is stable in solution at 37°C and compatible with CSF. Each mL of GABLOFEN contains baclofen USP 50 mcg, 500 mcg, 1,000 mcg or 2,000 mcg and sodium chloride 9 mg in Water for Injection; pH range is 5.5 to 7.5.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism of action of baclofen as a muscle relaxant and antispasticity agent is not fully understood. Baclofen inhibits both monosynaptic and polysynaptic ...
12.1 Mechanism of Action
The precise mechanism of action of baclofen as a muscle relaxant and antispasticity agent is not fully understood. Baclofen inhibits both monosynaptic and polysynaptic reflexes at the spinal level, possibly by decreasing excitatory neurotransmitter release from primary afferent terminals, although actions at supraspinal sites may also occur and contribute to its clinical effect. Baclofen is a structural analog of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), and may exert its effects by stimulation of the GABAB receptor subtype.
Baclofen when introduced directly into the intrathecal space permits effective CSF concentrations to be achieved with resultant plasma concentrations 100 times less than those occurring with oral administration. In people, as well as in animals, baclofen has been shown to have general CNS depressant properties as indicated by the production of sedation with tolerance, somnolence, ataxia, and respiratory and cardiovascular depression.12.2 Pharmacodynamics
Intrathecal Bolus
Adult Patients
The onset of action is generally one-half hour to one hour after an intrathecal bolus. Peak spasmolytic effect is seen at approximately four hours after dosing and effects may last four to eight hours. Onset, peak response, and duration of action may vary with individual patients depending on the dose and severity of symptoms.
Pediatric Patients
The onset, peak response and duration of action are similar to those seen in adult patients.
Continuous Infusion
Adult Patients
Intrathecal baclofen's antispastic action is first seen at 6 to 8 hours after initiation of continuous infusion. Maximum activity is observed in 24 to 48 hours.
Pediatric Patients
No additional information on continuous infusions is available for pediatric patients.Close12.3 Pharmacokinetics
The pharmacokinetics of cerebrospinal fluid (CSF) clearance of intrathecal baclofen calculated from intrathecal bolus or continuous infusion studies approximates CSF turnover, suggesting elimination is by bulk-flow removal of CSF.
Intrathecal Bolus
After a bolus lumbar injection of 50 mcg or 100 mcg intrathecal baclofen in seven patients, the average CSF elimination half-life was 1.51 hours over the first four hours and the average CSF clearance was approximately 30 mL/hour.
Continuous Infusion
The mean CSF clearance for intrathecal baclofen was approximately 30 mL/hour in a study involving ten patients on continuous intrathecal infusion. Concurrent plasma concentrations of baclofen during intrathecal administration are expected to be low (0 to 5 ng/mL). Limited pharmacokinetic data suggest that a lumbar-cisternal concentration gradient of about 4:1 is established along the neuroaxis during baclofen infusion. This is based upon simultaneous CSF sampling via cisternal and lumbar tap in 5 patients receiving continuous baclofen infusion at the lumbar level at doses associated with therapeutic efficacy; the interpatient variability was great. The gradient was not altered by position. Six pediatric patients (age 8 to 18 years) receiving continuous intrathecal baclofen infusion at doses of 77 to 400 mcg/day had plasma baclofen levels near or below 10 ng/mL. -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No increase in tumors was seen in rats receiving baclofen orally for two years. Mutagenesis - Mutagenicity assays ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No increase in tumors was seen in rats receiving baclofen orally for two years.
Mutagenesis
Mutagenicity assays with baclofen have not been performed.
Impairment of Fertility
Studies to assess the potential for adverse effects of baclofen on fertility have not been conducted.
-
14 CLINICAL STUDIESSpasticity of Spinal Cord Origin - Evidence supporting the efficacy of intrathecal baclofen was obtained in randomized, controlled investigations that compared the effects of either a single ...
Spasticity of Spinal Cord Origin
Evidence supporting the efficacy of intrathecal baclofen was obtained in randomized, controlled investigations that compared the effects of either a single intrathecal dose or a three day intrathecal infusion of intrathecal baclofen to placebo in patients with severe spasticity and spasms due to either spinal cord trauma or multiple sclerosis. Intrathecal baclofen was superior to placebo on both principal outcome measures employed: change from baseline in the Ashworth rating of spasticity and the frequency of spasms.
Close
Spasticity of Cerebral Origin
The efficacy of intrathecal baclofen was investigated in three controlled clinical trials; two enrolled patients with cerebral palsy and one enrolled patients with spasticity due to previous brain injury. The first study, a randomized controlled cross-over trial of 51 patients with cerebral palsy, provided strong, statistically significant results; intrathecal baclofen was superior to placebo in reducing spasticity as measured by the Ashworth Scale. A second cross-over study was conducted in 11 patients with spasticity arising from brain injury. Despite the small sample size, the study yielded a nearly significant test statistic (p=0.066) and provided directionally favorable results. The last study, however, did not provide data that could be reliably analyzed. -
16 HOW SUPPLIED/STORAGE AND HANDLINGGABLOFEN (baclofen injection) is a sterile, pyrogen-free, isotonic solution available in a single-dose syringe of 1 mL containing 50 mcg (50 mcg/mL) and in single-dose syringes and vials of ...
GABLOFEN (baclofen injection) is a sterile, pyrogen-free, isotonic solution available in a single-dose syringe of 1 mL containing 50 mcg (50 mcg/mL) and in single-dose syringes and vials of 10,000 mcg per 20 mL (500 mcg/mL), 20,000 mcg per 20 mL (1,000 mcg/mL), and 40,000 mcg per 20 mL (2,000 mcg/mL) for intrathecal administration only.
50 mcg per mL
NDC 66794-151-01: 1 mL Syringe – 50 mcg per 1 mL
500 mcg per mL
NDC 66794-155-01: 20 mL Syringe – 10,000 mcg per 20 mL
NDC 66794-155-02: 20 mL Vial – 10,000 mcg per 20 mL
1,000 mcg per mL
NDC 66794-156-01: 20 mL Syringe – 20,000 mcg per 20 mL
NDC 66794-156-02: 20 mL Vial – 20,000 mcg per 20 mL
2,000 mcg per mL
NDC 66794-157-01: 20 mL Syringe – 40,000 mcg per 20 mL
NDC 66794-157-02: 20 mL Vial – 40,000 mcg per 20 mL
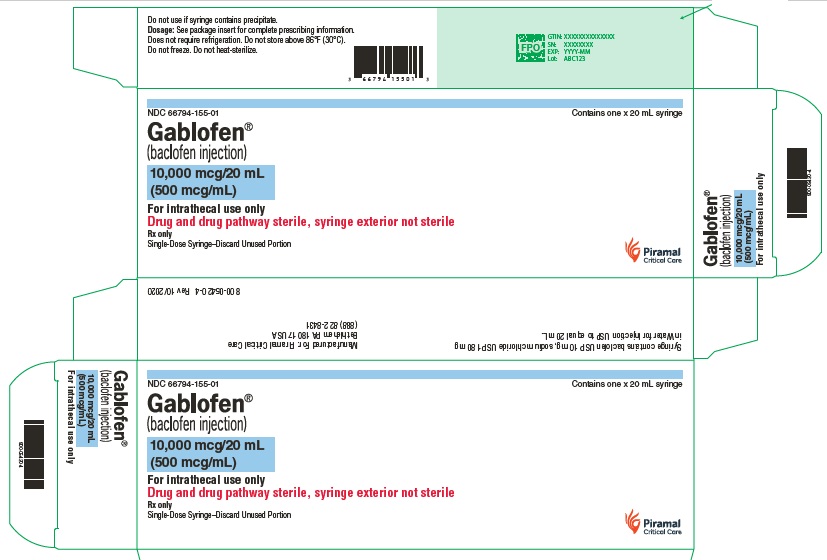
GABLOFEN does not contain any antioxidants, preservatives or other potentially neurotoxic additives. Each single-dose vial or syringe is intended for single use only. Discard any unused portion.
Does not require refrigeration.
Do not store above 86°F (30°C).
Do not freeze.
Do not heat sterilize.
Close -
17 PATIENT COUNSELING INFORMATIONRisks Related to Sudden Withdrawal of GABLOFEN - Advise patients and caregivers that sudden withdrawal of GABLOFEN, regardless of the cause, can result in serious complications that include high ...
Risks Related to Sudden Withdrawal of GABLOFEN
Advise patients and caregivers that sudden withdrawal of GABLOFEN, regardless of the cause, can result in serious complications that include high fever, confusion, muscle stiffness, multiple organ-system failure, and death. Inform patients that early symptoms of GABLOFEN withdrawal may include increased spasticity, itching, and tingling of extremities. If GABLOFEN withdrawal or a pump malfunction is suspected, patients should be brought immediately to a hospital for assessment and treatment.
Inform patients and caregivers that sudden withdrawal occurs most frequently due to a delivery problem with the catheter or the pump, or failure to refill the pump on schedule. Advise patients and their caregivers to pay careful attention to infusion system alarms. Instruct patients and caregivers that if they miss their scheduled pump refill, they should immediately contact their physician to reschedule the refill before the pump runs out of drug.
GABLOFEN Overdose
Inform patients and their caregivers that GABLOFEN overdose may occur suddenly or insidiously, and that symptoms may include confusion, drowsiness, lightheadedness, dizziness, slow or shallow breathing, seizures, loss of muscle tone, loss of consciousness, and coma. If an overdose appears likely, patients should be brought immediately to a hospital for assessment and possible emptying of the pump.
Operation of Automobiles and Other Dangerous Machinery
Advise patients that GABLOFEN may cause drowsiness, and that they should exercise caution regarding the operation of automobiles or other dangerous machinery, or activities made hazardous by decreased alertness.
Increased Risk of Drowsiness with Alcohol and Other CNS Depressants
Inform patients and their caregivers that the drowsiness associated with GABLOFEN use can be worsened by alcohol and other CNS depressants. Advise patients to read all medicine labels carefully, and to tell their physician about all prescription and nonprescription drugs they may use.
Distributed By:
Piramal Critical Care, Inc.
3950 Schelden Circle
Bethlehem, PA 18017, USA
Phone: 888 822 8431
Issued 10/2020
Close -
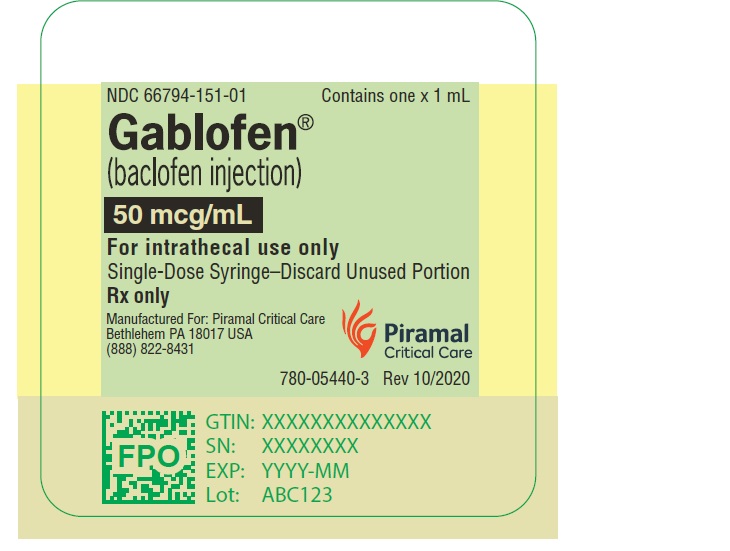
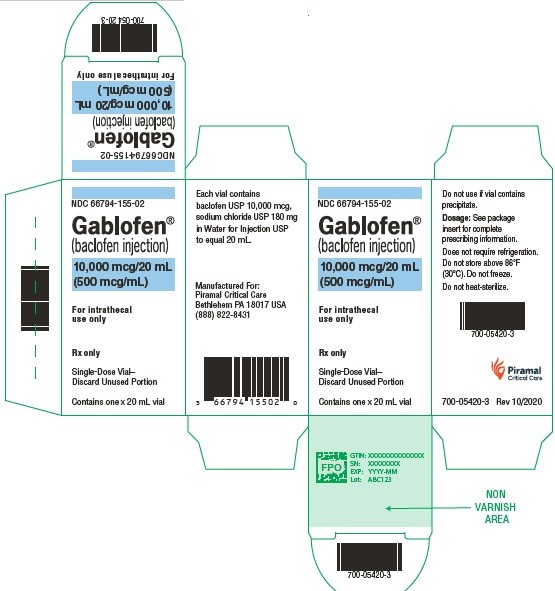
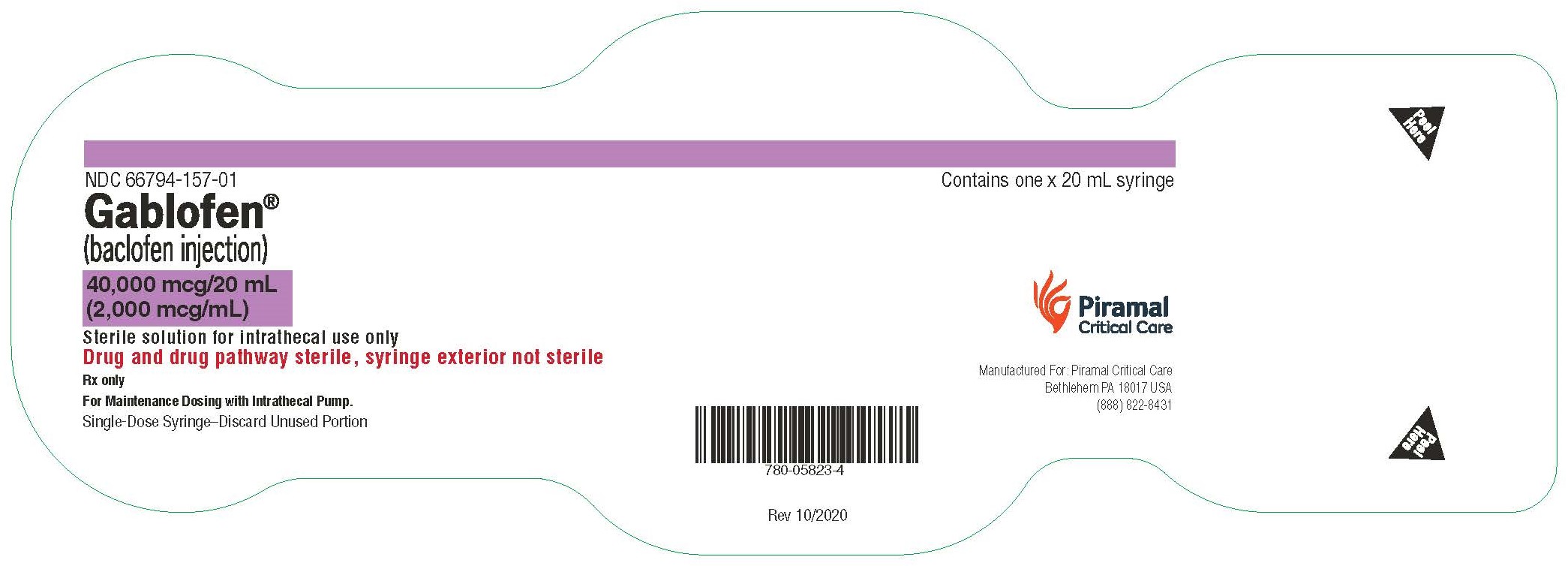
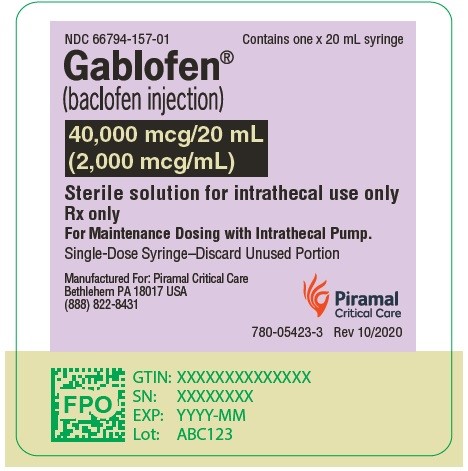
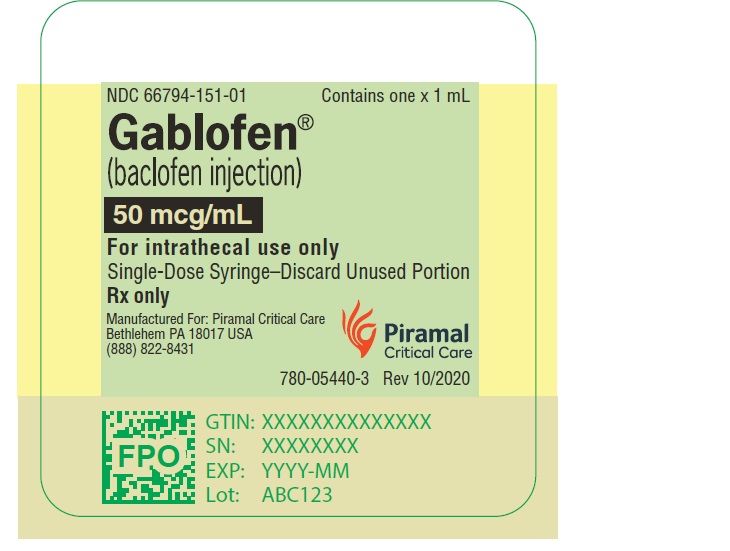
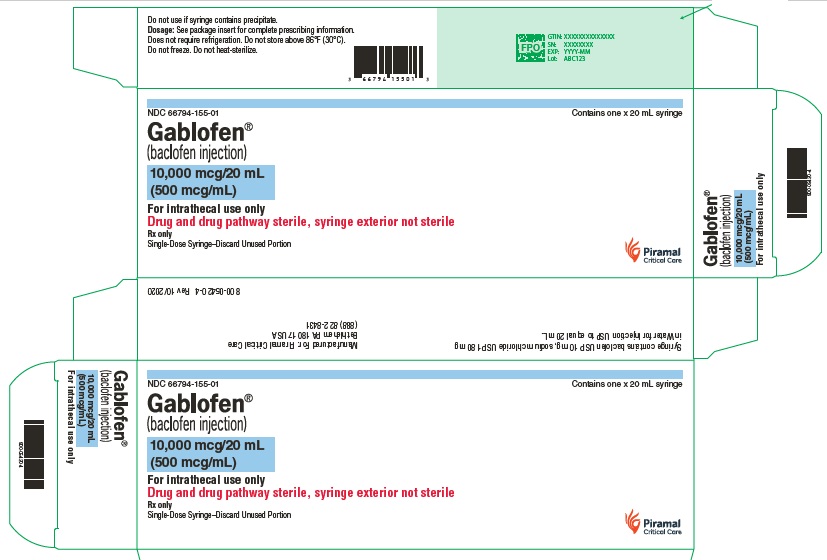
PRINCIPAL DISPLAY PANELGablofen® (Baclofen injection) 50 mcg/mL - For intrathecal use only - NDC 66794-151-01 - Contains one x 1 mL - Gablofen® (baclofen injection) 10,000 mcg/20 mL - (500 mcg/mL) Sterile ...
Gablofen®
(Baclofen injection)
50 mcg/mL
For intrathecal use only
NDC 66794-151-01
Contains one x 1 mL

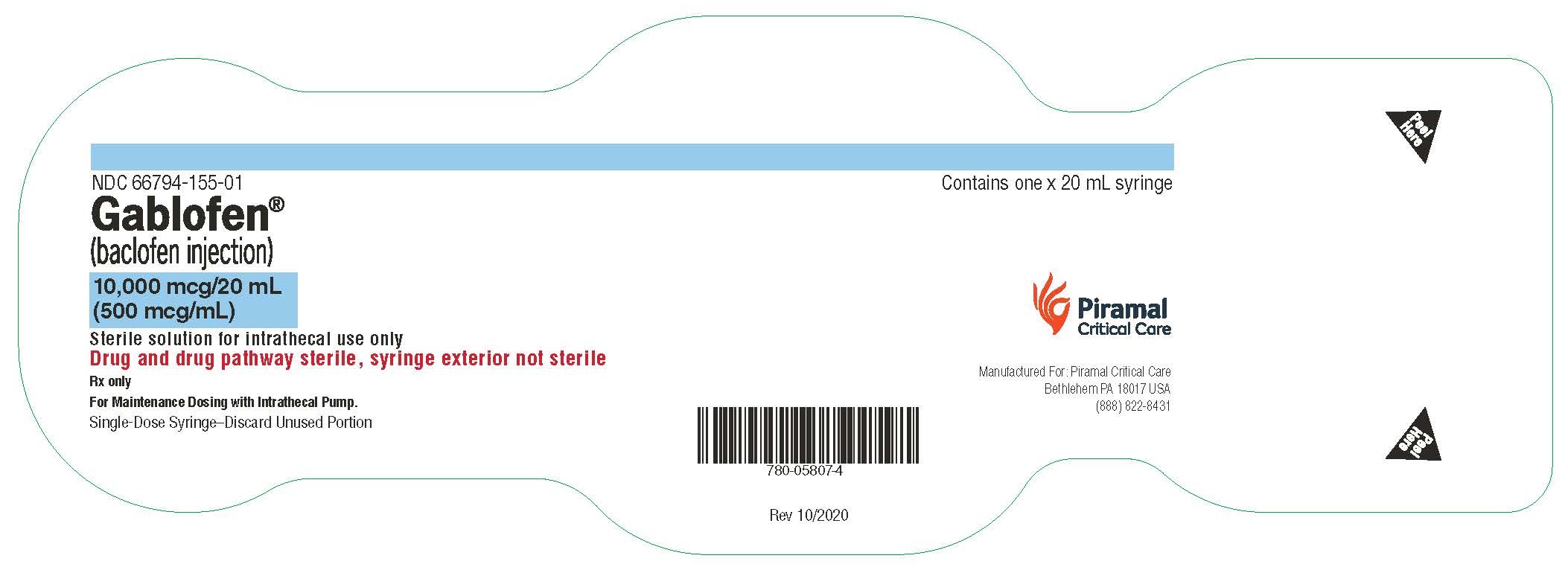
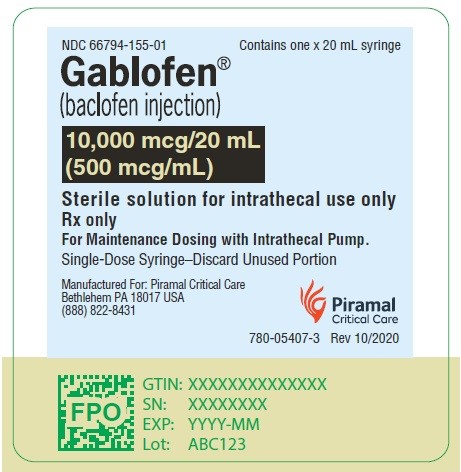
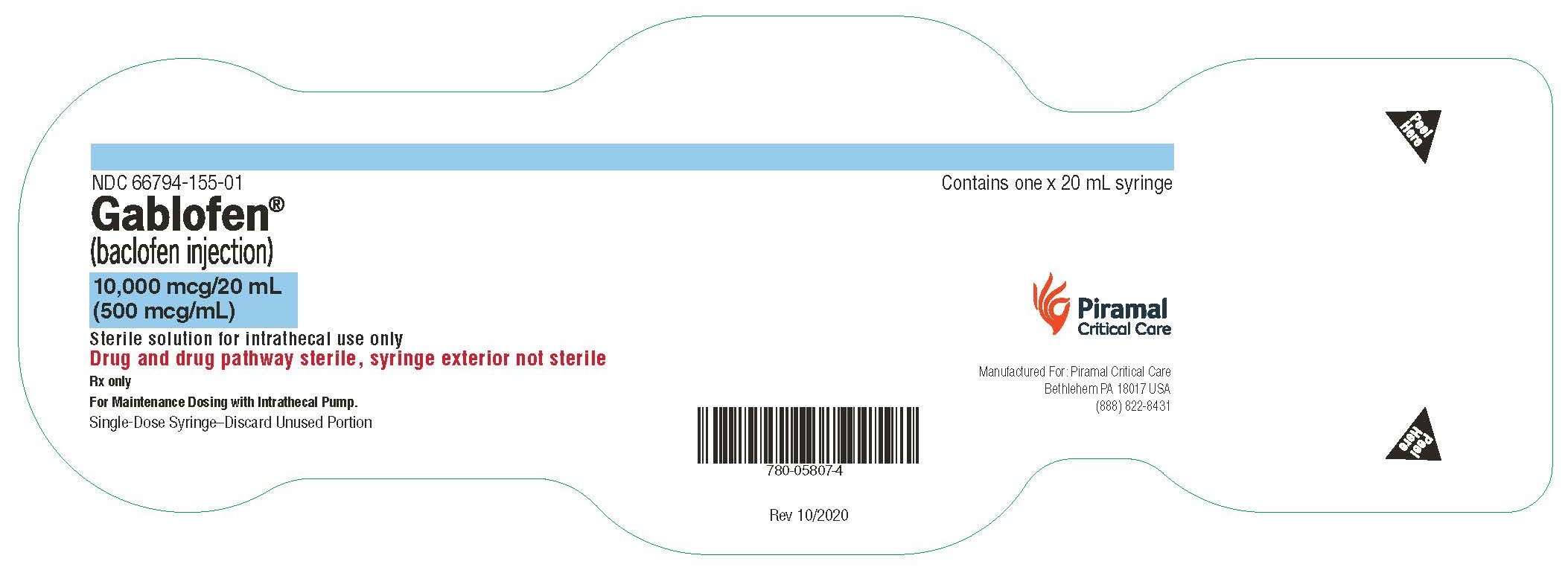
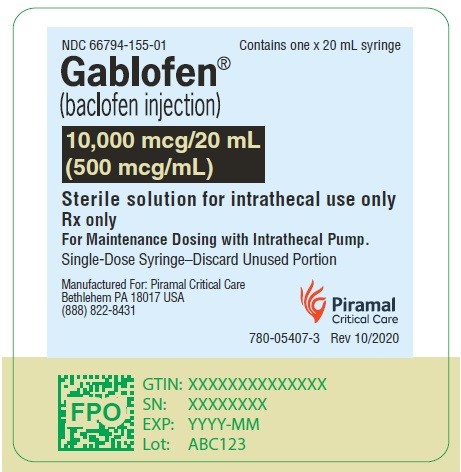
Gablofen® (baclofen injection)
10,000 mcg/20 mL
(500 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-155-01
Contains one x 20 mL syringe
Gablofen® (baclofen injection)
10,000 mcg/20 mL
(500 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-155-02

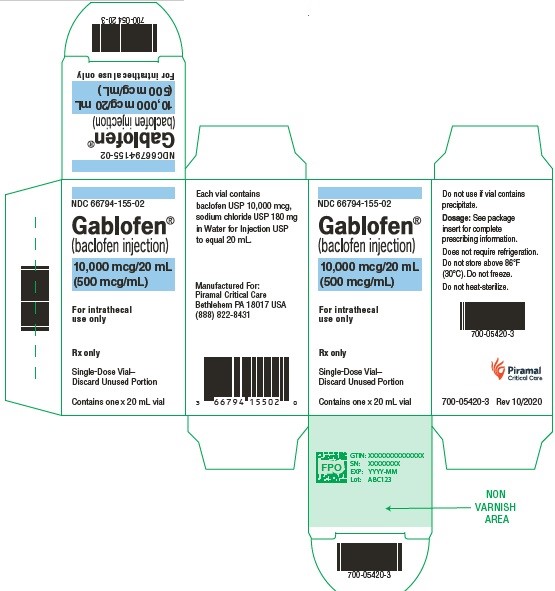


Gablofen®
(baclofen injection)
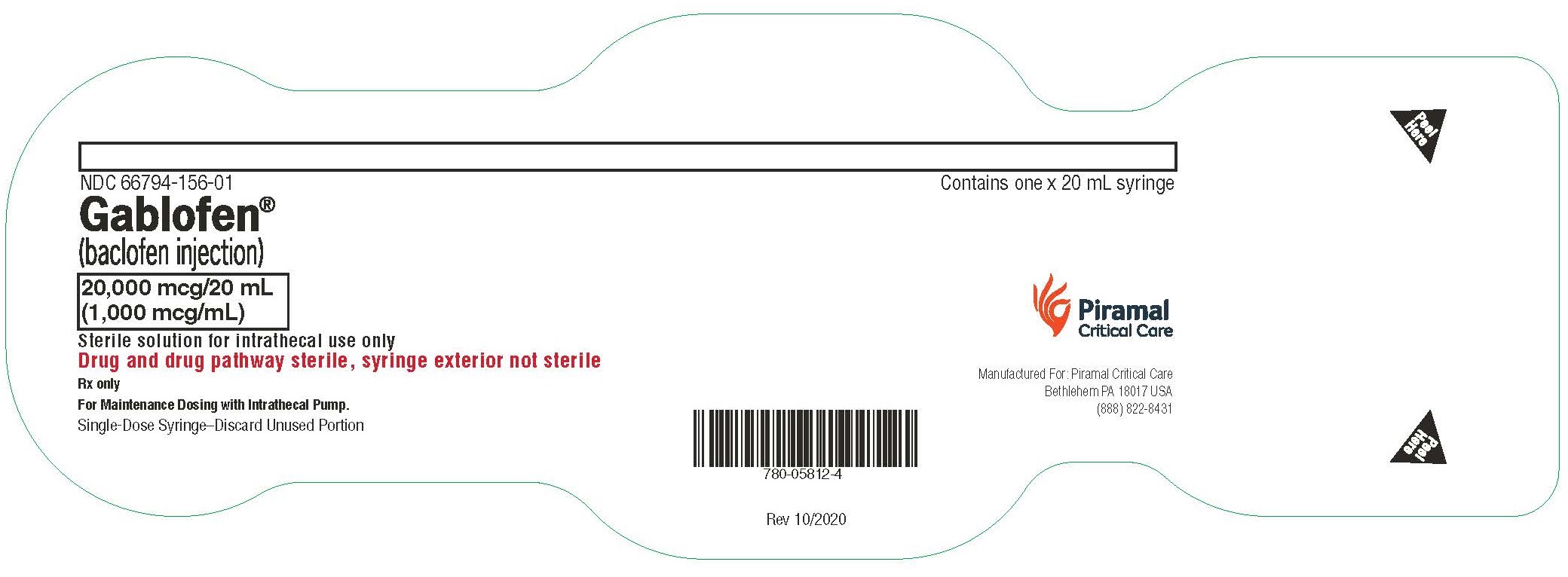
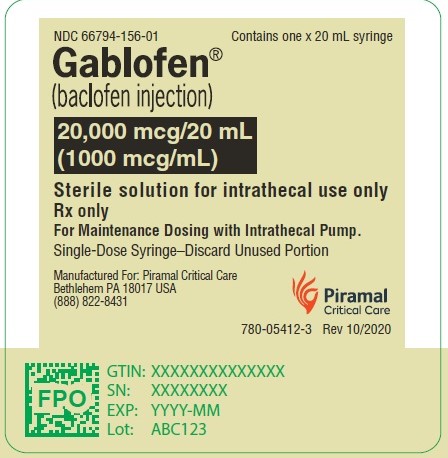
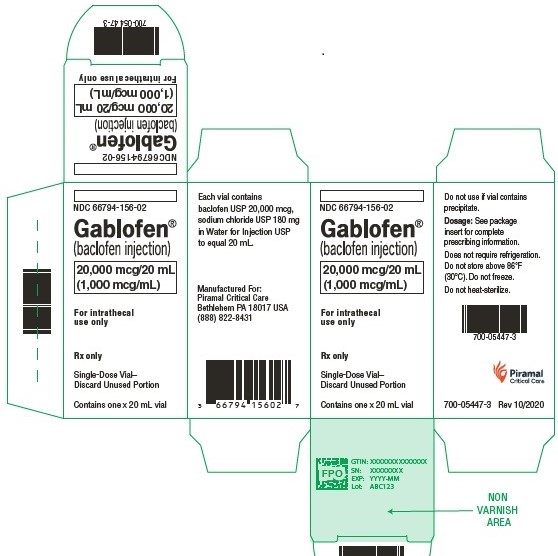
20,000 mcg/20 mL
(1,000 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-156-01
Contains one x 20 mL syringe
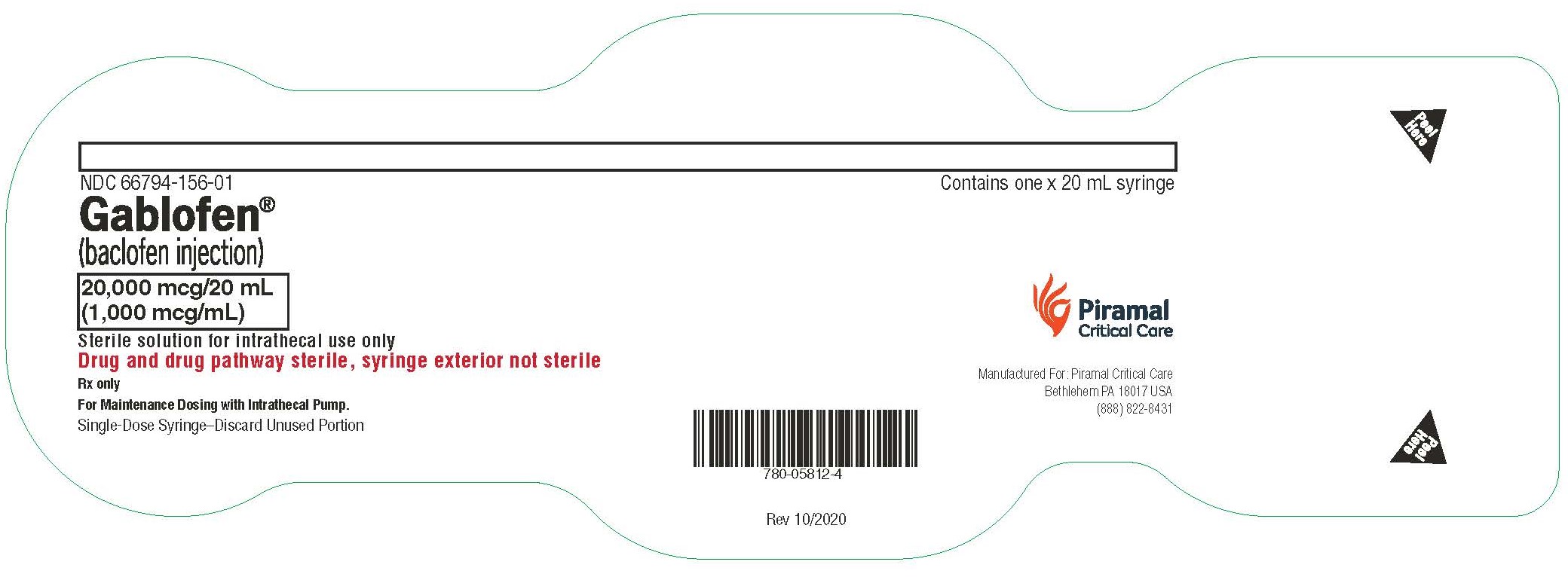
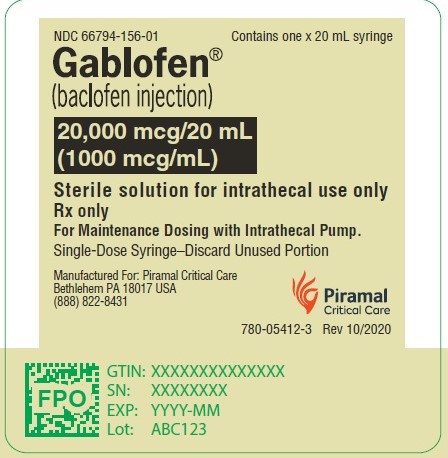

Gablofen®
(baclofen injection)
20,000 mcg/20 mL
(1,000 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-156-02

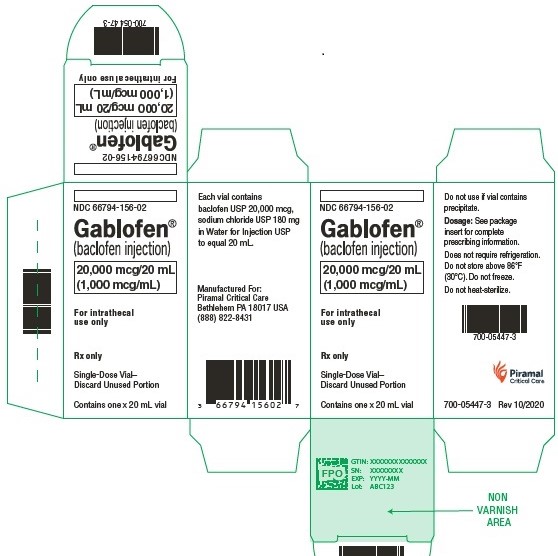


Gablofen® (baclofen injection)
40,000 mcg/20 mL
(2,000 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-157-01
Contains one x 20 mL syringe
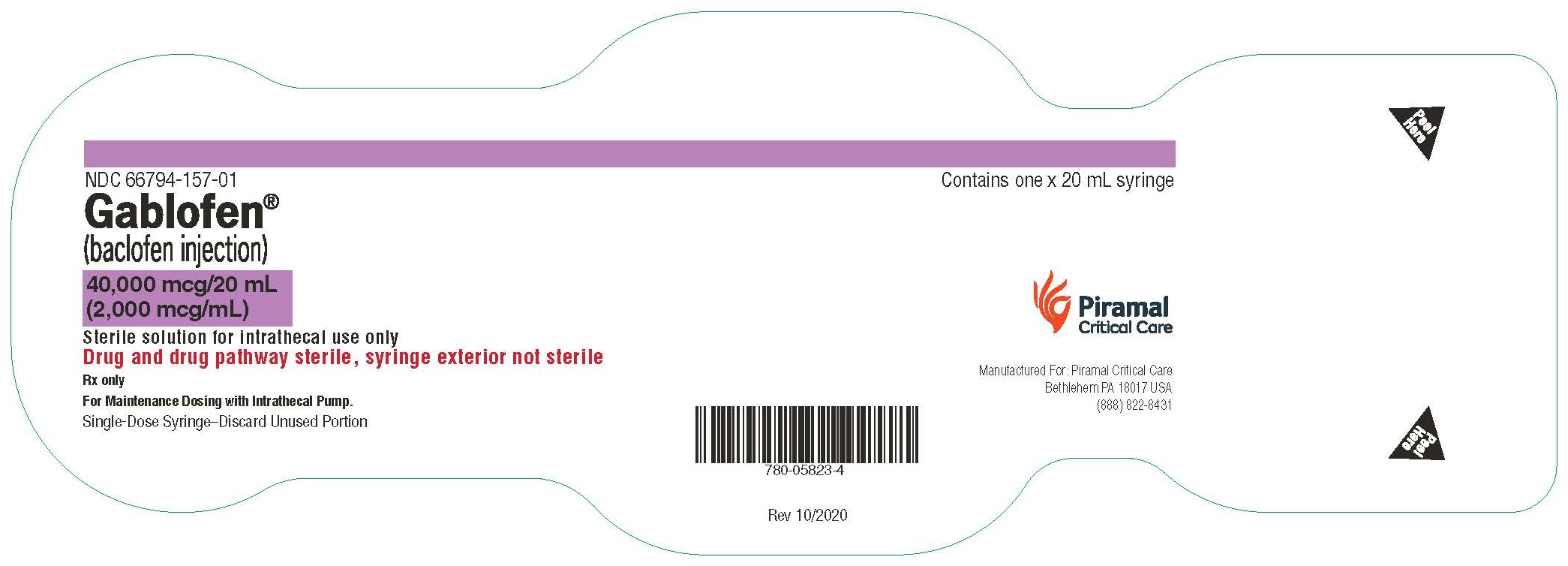
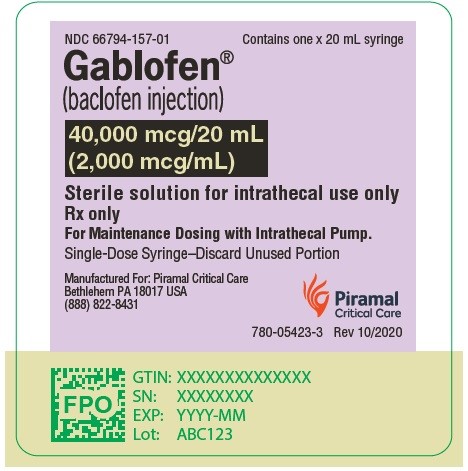

Gablofen® (baclofen injection)
40,000 mcg/20 mL
(2,000 mcg/mL)
Sterile solution for intrathecal use only
NDC 66794-157-02

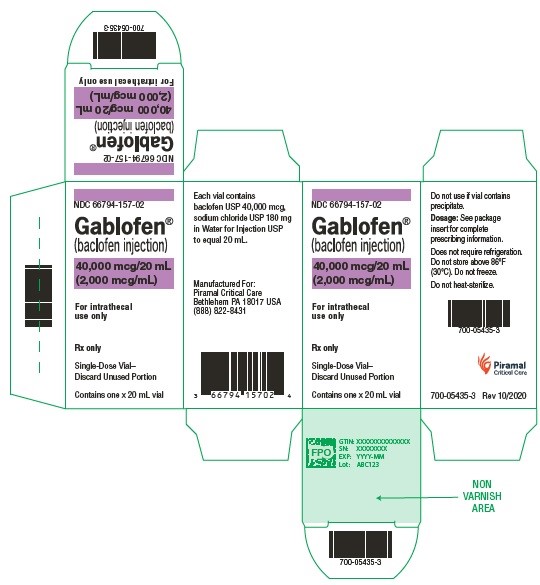

Close
-
INGREDIENTS AND APPEARANCEProduct Information
GABLOFEN baclofen injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66794-151 Route of Administration INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 50 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66794-151-01 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 04/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022462 04/01/2017 GABLOFEN baclofen injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66794-155 Route of Administration INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 500 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66794-155-01 20 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 04/01/2017 2 NDC:66794-155-02 20 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 04/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022462 04/01/2017 GABLOFEN baclofen injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66794-156 Route of Administration INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 1000 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66794-156-01 20 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 04/01/2017 2 NDC:66794-156-02 20 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 04/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022462 04/01/2017 GABLOFEN baclofen injection injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66794-157 Route of Administration INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACLOFEN (UNII: H789N3FKE8) (BACLOFEN - UNII:H789N3FKE8) BACLOFEN 2000 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66794-157-01 20 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 04/01/2017 2 NDC:66794-157-02 20 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 04/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022462 04/01/2017 Labeler - Piramal Critical Care Inc (805600439) Registrant - Piramal Critical Care Inc (805600439) Establishment Name Address ID/FEI Business Operations Cangene BioPharma dba Bora Pharmaceuticals Injectables Inc 119352788 analysis(66794-156, 66794-155, 66794-157, 66794-151) , manufacture(66794-151, 66794-155, 66794-156, 66794-157) , label(66794-151, 66794-155, 66794-156, 66794-157) , pack(66794-151, 66794-155, 66794-156, 66794-157) Establishment Name Address ID/FEI Business Operations PCAS Finland Oy 369587311 api manufacture(66794-151, 66794-155, 66794-156, 66794-157)
CloseEstablishment Name Address ID/FEI Business Operations Gland Pharma Limited 858971074 analysis(66794-151, 66794-155, 66794-156, 66794-157) , label(66794-151, 66794-155, 66794-156, 66794-157) , manufacture(66794-151, 66794-155, 66794-156, 66794-157) , pack(66794-151, 66794-155, 66794-156, 66794-157)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
GABLOFEN- baclofen injection injection, solution
Number of versions: 12
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 31, 2025 | 39 (current) | download |
| Dec 20, 2024 | 38 | download |
| Sep 9, 2024 | 37 | download |
| Jan 2, 2023 | 36 | download |
| Jun 27, 2022 | 35 | download |
| Dec 3, 2021 | 34 | download |
| Apr 29, 2021 | 33 | download |
| Oct 23, 2020 | 32 | download |
| Oct 21, 2020 | 31 | download |
| Dec 27, 2019 | 30 | download |
| Jan 26, 2018 | 29 | download |
| Apr 5, 2017 | 1 | download |
RxNorm
GABLOFEN- baclofen injection injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 308517 | baclofen 10 MG in 20 ML (500 MCG/ML) Injection | PSN |
| 2 | 308517 | 20 ML baclofen 0.5 MG/ML Injection | SCD |
| 3 | 308517 | baclofen 10,000 MCG per 20 ML Injection | SY |
| 4 | 1047437 | baclofen 50 MCG in 1 ML Prefilled Syringe | PSN |
| 5 | 1047437 | 1 ML baclofen 0.05 MG/ML Prefilled Syringe | SCD |
| 6 | 1047437 | baclofen 50 MCG per 1 ML Prefilled Syringe | SY |
| 7 | 1047440 | Gablofen 50 MCG in 1 ML Prefilled Syringe | PSN |
| 8 | 1047440 | 1 ML baclofen 0.05 MG/ML Prefilled Syringe [Gablofen] | SBD |
| 9 | 1047440 | 1 ML Gablofen 0.05 MG/ML Prefilled Syringe | SY |
| 10 | 1047440 | Gablofen 50 MCG per 1 ML Prefilled Syringe | SY |
| 11 | 1047444 | Gablofen 10,000 MCG in 20 ML (500 MCG/ML) Injection | PSN |
| 12 | 1047444 | 20 ML baclofen 0.5 MG/ML Injection [Gablofen] | SBD |
| 13 | 1047444 | 20 ML Gablofen 0.5 MG/ML Injection | SY |
| 14 | 1047444 | Gablofen 10,000 MCG per 20 ML Injection | SY |
| 15 | 1047444 | Gablofen 500 MCG per 1 ML Injection | SY |
| 16 | 1300890 | baclofen 20,000 MCG in 20 ML (1,000 MCG/ML) Injection | PSN |
| 17 | 1300890 | 20 ML baclofen 1 MG/ML Injection | SCD |
| 18 | 1300890 | baclofen 20,000 MCG per 20 ML Injection | SY |
| 19 | 1301627 | Gablofen 20,000 MCG in 20 ML (1,000 MCG/ML) Injection | PSN |
| 20 | 1301627 | 20 ML baclofen 1 MG/ML Injection [Gablofen] | SBD |
| 21 | 1301627 | 20 ML Gablofen 1 MG/ML Injection | SY |
| 22 | 1301627 | Gablofen 20,000 MCG per 20 ML Injection | SY |
| 23 | 1369783 | baclofen 10,000 MCG in 20 ML (500 MCG/ML) Prefilled Syringe | PSN |
| 24 | 1369783 | 20 ML baclofen 0.5 MG/ML Prefilled Syringe | SCD |
| 25 | 1369783 | baclofen 10,000 MCG per 20 ML Prefilled Syringe | SY |
| 26 | 1369784 | Gablofen 10,000 MCG in 20 ML (500 MCG/ML) Prefilled Syringe | PSN |
| 27 | 1369784 | 20 ML baclofen 0.5 MG/ML Prefilled Syringe [Gablofen] | SBD |
| 28 | 1369784 | 20 ML Gablofen 0.5 MG/ML Prefilled Syringe | SY |
| 29 | 1369784 | Gablofen 10,000 MCG per 20 ML Prefilled Syringe | SY |
| 30 | 1369787 | baclofen 20,000 MCG in 20 ML (1,000 MCG/ML) Prefilled Syringe | PSN |
| 31 | 1369787 | 20 ML baclofen 1 MG/ML Prefilled Syringe | SCD |
| 32 | 1369787 | baclofen 20,000 MCG per 20 ML Prefilled Syringe | SY |
| 33 | 1369788 | Gablofen 20,000 MCG in 20 ML (1,000 MCG/ML) Prefilled Syringe | PSN |
| 34 | 1369788 | 20 ML baclofen 1 MG/ML Prefilled Syringe [Gablofen] | SBD |
| 35 | 1369788 | 20 ML Gablofen 1 MG/ML Prefilled Syringe | SY |
| 36 | 1369788 | Gablofen 20,000 MCG per 20 ML Prefilled Syringe | SY |
| 37 | 1369791 | baclofen 40,000 MCG in 20 ML (2,000 MCG/ML) Prefilled Syringe | PSN |
| 38 | 1369791 | 20 ML baclofen 2 MG/ML Prefilled Syringe | SCD |
| 39 | 1369791 | baclofen 2 MG/ML 20 ML Prefilled Syringe | SY |
| 40 | 1369791 | baclofen 40,000 MCG per 20 ML Prefilled Syringe | SY |
| 41 | 1369792 | Gablofen 40,000 MCG in 20 ML (2,000 MCG/ML) Prefilled Syringe | PSN |
| 42 | 1369792 | 20 ML baclofen 2 MG/ML Prefilled Syringe [Gablofen] | SBD |
| 43 | 1369792 | 20 ML Gablofen 2 MG/ML Prefilled Syringe | SY |
| 44 | 1369792 | Gablofen 2 MG/ML 20 ML Prefilled Syringe | SY |
| 45 | 1369792 | Gablofen 40,000 mcg per 20 ML Prefilled Syringe | SY |
| 46 | 1666613 | baclofen 40 MG in 20 ML (2,000 MCG/ML) Injection | PSN |
| 47 | 1666613 | 20 ML baclofen 2 MG/ML Injection | SCD |
| 48 | 1666613 | baclofen 40,000 MCG per 20 ML Injection | SY |
| 49 | 1666615 | Gablofen 40,000 MCG in 20 ML (2,000 MCG/ML) Injection | PSN |
| 50 | 1666615 | 20 ML baclofen 2 MG/ML Injection [Gablofen] | SBD |
| 51 | 1666615 | 20 ML Gablofen 2 MG/ML Injection | SY |
| 52 | 1666615 | Gablofen 40,000 MCG per 20 ML Injection | SY |
Get Label RSS Feed for this Drug
GABLOFEN- baclofen injection injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=00d3e846-dd92-448d-9ab8-6a07be823cc1
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
GABLOFEN- baclofen injection injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 66794-151-01 |
| 2 | 66794-155-01 |
| 3 | 66794-155-02 |
| 4 | 66794-156-01 |
| 5 | 66794-156-02 |
| 6 | 66794-157-01 |
| 7 | 66794-157-02 |