Label: ZIPRASIDONE capsule
- NDC Code(s): 16714-835-01, 16714-836-01, 16714-837-01, 16714-838-01
- Packager: NorthStar Rx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZIPRASIDONE CAPSULES safely and effectively. See full prescribing information for ZIPRASIDONE CAPSULES. ZIPRASIDONE capsules ...These highlights do not include all the information needed to use ZIPRASIDONE CAPSULES safely and effectively. See full prescribing information for ZIPRASIDONE CAPSULES.
ZIPRASIDONE capsules, for oral use
Initial U.S. Approval: 2001WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Ziprasidone is not approved for the treatment of patients with dementia-related psychosis (5.1)
INDICATIONS AND USAGE
Ziprasidone capsule is an atypical antipsychotic. In choosing among treatments, prescribers should be aware of the capacity of ziprasidone capsule to prolong the QT interval and may consider the use of other drugs first (1)
Ziprasidone capsules are indicated for the:
• treatment of schizophrenia in adults. (1)
• acute treatment of adults as monotherapy of manic or mixed episodes associated with bipolar I disorder. (1)
• maintenance treatment of bipolar I disorder as an adjunct to lithium or valproate in adults. (1)
DOSAGE AND ADMINISTRATION
- Administer capsules orally with food. Do not open, crush, or chew. (2.1)
- Schizophrenia: Initiate at 20 mg twice daily. Daily dosage may be adjusted up to 80 mg twice daily. Dose adjustments should occur at intervals of not less than 2 days. Safety and efficacy has been demonstrated in doses up to 100 mg twice daily. The lowest effective dose should be used. (2.2)
- Acute treatment of manic/mixed episodes of bipolar I disorder: Initiate at 40 mg twice daily. Increase to 60 mg or 80 mg twice daily on day 2 of treatment. Subsequent dose adjustments should be based on tolerability and efficacy within the range of 40 to 80 mg twice daily. (2.3)
- Maintenance treatment of bipolar I disorder as an adjunct to lithium or valproate: Continue treatment at the same dose on which the patient was initially stabilized, within the range of 40 to 80 mg twice daily. (2.3)
DOSAGE FORMS AND STRENGTHS
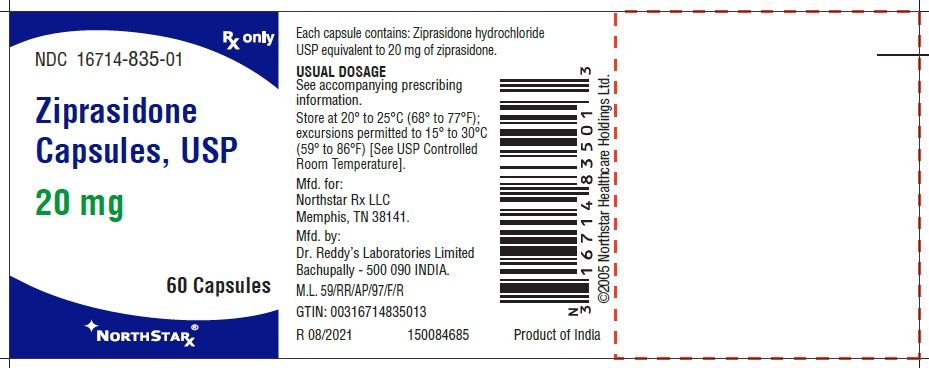
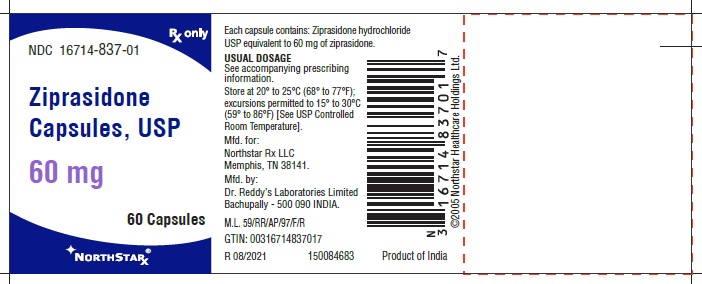
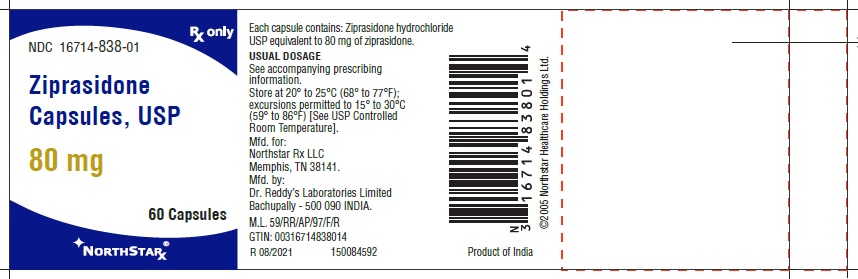
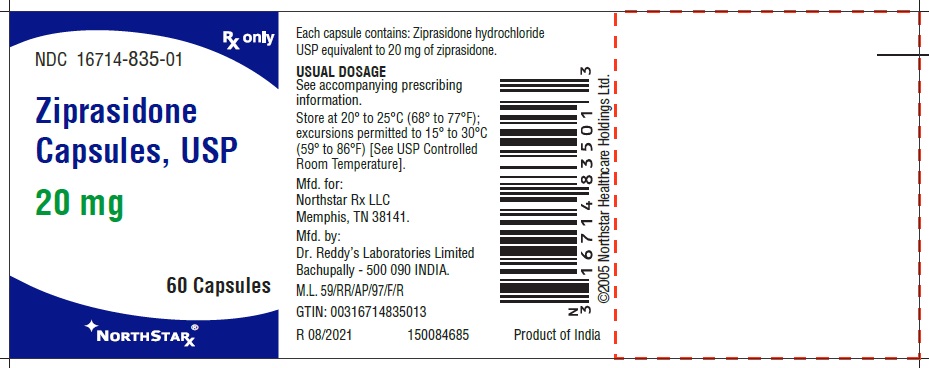
- Capsules: 20 mg, 40 mg, 60 mg, and 80 mg (3)
CONTRAINDICATIONS
- Do not use in patients with a known history of QT prolongation (4.1)
- Do not use in patients with recent acute myocardial infarction (4.1)
- Do not use in patients with uncompensated heart failure (4.1)
- Do not use in combination with other drugs that have demonstrated QT prolongation (4.1)
- Do not use in patients with known hypersensitivity to ziprasidone (4.2)
WARNINGS AND PRECAUTIONS
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack). (5.2)
- QT Interval Prolongation: Ziprasidone use should be avoided in patients with bradycardia, hypokalemia or hypomagnesemia, congenital prolongation of the QT interval, or in combination with other drugs that have demonstrated QT prolongation. (5.3)
- Neuroleptic Malignant Syndrome (NMS): Potentially fatal symptom complex has been reported with antipsychotic drugs. Manage with immediate discontinuation of drug and close monitoring. (5.4)
- Severe Cutaneous Adverse Reactions, such as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) and Stevens-Johnson syndrome has been reported with ziprasidone exposure. DRESS and other Severe Cutaneous Adverse Reactions (SCAR) are sometimes fatal. Discontinue ziprasidone if DRESS or SCAR are suspected.(5.5)
- Tardive Dyskinesia: May develop acutely or chronically (5.6)
- Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/ cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and weight gain. (5.7)
- Hyperglycemia and Diabetes Mellitus (DM): Monitor all patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients with DM risk factors should undergo blood glucose testing before and during treatment. (5.7)
- Dyslipidemia: Undesirable alterations have been observed in patients treated with atypical antipsychotics. (5.7)
- Weight Gain: Weight gain has been reported. Monitor weight gain. (5.7)
- Rash: Discontinue in patients who develop a rash without an identified cause.(5.8)
- Orthostatic Hypotension: Use with caution in patients with known cardiovascular or cerebrovascular disease. (5.9)
- Leukopenia, Neutropenia, and Agranulocytosis has been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue ziprasidone at the first sign of a decline in WBC in the absence of other causative factors. (5.11)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower seizure threshold. (5.12)
- Potential for Cognitive and Motor impairment: Patients should use caution when operating machinery. (5.13)
- Suicide: Closely supervise high-risk patients. (5.18)
ADVERSE REACTIONS
Commonly observed adverse reactions (incidence ≥5% and at least twice the incidence for placebo) were:
• Schizophrenia: Somnolence, respiratory tract infection. (6.1)
• Manic and Mixed Episodes Associated with Bipolar Disorder: Somnolence, extrapyramidal symptoms, dizziness, akathisia, abnormal vision, asthenia, vomiting (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact NorthStar Rx LLC at 1-800-206-7821 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information for Ziprasidone Capsules
2.2 Schizophrenia
2.3 Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 QT Prolongation
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients With Dementia-Related Psychosis
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients With Dementia-Related Psychosis
5.3 QT Prolongation and Risk of Sudden Death
5.4 Neuroleptic Malignant Syndrome (NMS)
5.5 Severe Cutaneous Adverse Reactions
5.6 Tardive Dyskinesia
5.7 Metabolic Changes
5.8 Rash
5.9 Orthostatic Hypotension
5.10 Falls
5.11 Leukopenia, Neutropenia, and Agranulocytosis
5.12 Seizures
5.13 Dysphagia
5.14 Hyperprolactinemia
5.15 Potential for Cognitive and Motor Impairment
5.16 Priapism
5.17 Body Temperature Regulation
5.18 Suicide
5.19 Patients With Concomitant Illnesses
5.20 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Metabolic Pathway
7.2 In Vitro Studies
7.3 Pharmacodynamic Interactions
7.4 Pharmacokinetic Interactions
7.5 Lithium
7.6 Oral Contraceptives
7.7 Dextromethorphan
7.8 Valproate
7.9 Other Concomitant Drug Therapy
7.10 Food Interaction
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Age and Gender Effects
8.9 Smoking
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Schizophrenia
14.2 Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Ziprasidone is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEZiprasidone capsules are indicated for the treatment of schizophrenia, as monotherapy for the acute treatment of bipolar manic or mixed episodes, and as an adjunct to lithium or valproate for the ...
Ziprasidone capsules are indicated for the treatment of schizophrenia, as monotherapy for the acute treatment of bipolar manic or mixed episodes, and as an adjunct to lithium or valproate for the maintenance treatment of bipolar disorder. When deciding among the alternative treatments available for the condition needing treatment, the prescriber should consider the finding of ziprasidone’s greater capacity to prolong the QT/QTc interval compared to several other antipsychotic drugs [see Warnings and Precautions (5.3)]. Prolongation of the QTc interval is associated in some other drugs with the ability to cause torsade de pointes-type arrhythmia, a potentially fatal polymorphic ventricular tachycardia, and sudden death. In many cases this would lead to the conclusion that other drugs should be tried first. Whether ziprasidone will cause torsade de pointes or increase the rate of sudden death is not yet known [see Warnings and Precautions (5.3)]
Schizophrenia
- Ziprasidone capsules are indicated for the treatment of schizophrenia in adults [see Clinical Studies (14.1)].
Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate)
-
Ziprasidone capsules are indicated as monotherapy for the acute treatment of adults with manic or mixed episodes associated with bipolar I disorder [see Clinical Studies (14.2)].
-
Ziprasidone capsules are indicated as an adjunct to lithium or valproate for the maintenance treatment of bipolar I disorder in adults [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION2.1 Administration Information for Ziprasidone Capsules - Administer ziprasidone capsules orally with food. Swallow capsules whole, do not open, crush, or chew the capsules. 2.2 ...
2.1 Administration Information for Ziprasidone Capsules
Administer ziprasidone capsules orally with food. Swallow capsules whole, do not open, crush, or chew the capsules.
2.2 Schizophrenia
Dose Selection
Ziprasidone capsules should be administered at an initial daily dose of 20 mg twice daily with food. In some patients, daily dosage may subsequently be adjusted on the basis of individual clinical status up to 80 mg twice daily. Dosage adjustments, if indicated, should generally occur at intervals of not less than 2 days, as steady-state is achieved within 1 to 3 days. In order to ensure use of the lowest effective dose, patients should ordinarily be observed for improvement for several weeks before upward dosage adjustment.
Efficacy in schizophrenia was demonstrated in a dose range of 20 mg to 100 mg twice daily in short-term, placebo-controlled clinical trials. There were trends toward dose response within the range of 20 mg to 80 mg twice daily, but results were not consistent. An increase to a dose greater than 80 mg twice daily is not generally recommended. The safety of doses above 100 mg twice daily has not been systematically evaluated in clinical trials [see Clinical Studies (14.1)].
Maintenance Treatment
While there is no body of evidence available to answer the question of how long a patient treated with ziprasidone should remain on it, a maintenance study in patients who had been symptomatically stable and then randomized to continue ziprasidone or switch to placebo demonstrated a delay in time to relapse for patients receiving ziprasidone capsules [see Clinical Studies (14.1)]. No additional benefit was demonstrated for doses above 20 mg twice daily. Patients should be periodically reassessed to determine the need for maintenance treatment.
Close2.3 Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate)
Acute Treatment of Manic or Mixed Episodes
In adults oral ziprasidone should be administered at an initial daily dose of 40 mg twice daily with food. The dose may then be increased to 60 mg or 80 mg twice daily on the second day of treatment and subsequently adjusted on the basis of tolerance and efficacy within the range 40 mg to 80 mg twice daily. In the flexible-dose clinical trials, the mean daily dose administered was approximately 120 mg [see Clinical Studies (14.2)].
Maintenance Treatment (as an adjunct to lithium or valproate)
Continue treatment at the same dose on which the patient was initially stabilized, within the range of 40 mg to 80 mg twice daily with food. Patients should be periodically reassessed to determine the need for maintenance treatment [see Clinical Studies (14.2)].
-
3 DOSAGE FORMS AND STRENGTHSZiprasidone capsules, USP 20 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and Flesh opaque body, imprinted “RDY” on ...
Ziprasidone capsules, USP 20 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and Flesh opaque body, imprinted “RDY” on cap and “256” on body with black ink.
Ziprasidone capsules, USP 40 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and LT Turquoise blue opaque body, imprinted “RDY” on cap and “257” on body with black ink.
Ziprasidone capsules, USP 60 mg (ziprasidone), are light pink to brown granular powder filled in size “3” hard gelatin capsules having Flesh opaque cap and Flesh opaque body, imprinted “RDY” on cap and “258” on body with black ink.
Ziprasidone capsules, USP 80 mg (ziprasidone), are light pink to brown granular powder filled in size “2” hard gelatin capsules having LT Turquoise blue opaque cap and Flesh opaque body, imprinted “RDY” on cap and “259” on body with black ink.
Close -
4 CONTRAINDICATIONS4.1 QT Prolongation Because of ziprasidone’s dose-related prolongation of the QT interval and the known association of fatal arrhythmias with QT prolongation by some other drugs ...
4.1 QT Prolongation
Because of ziprasidone’s dose-related prolongation of the QT interval and the known association of fatal arrhythmias with QT prolongation by some other drugs, ziprasidone is contraindicated:
- in patients with a known history of QT prolongation (including congenital long QT syndrome)
- in patients with recent acute myocardial infarction
- in patients with uncompensated heart failure
Pharmacokinetic/pharmacodynamic studies between ziprasidone and other drugs that prolong the QT interval have not been performed. An additive effect of ziprasidone and other drugs that prolong the QT interval cannot be excluded. Therefore, ziprasidone should not be given with:
- dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, pimozide, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol or tacrolimus.
- other drugs that have demonstrated QT prolongation as one of their pharmacodynamic effects and have this effect described in the full prescribing information as a contraindication or a boxed or bolded warning [see Warnings and Precautions (5.3)].
Close4.2 Hypersensitivity
Ziprasidone is contraindicated in individuals with a known hypersensitivity to the product.
-
5 WARNINGS AND PRECAUTIONS5.1 Increased Mortality in Elderly Patients With Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of ...
5.1 Increased Mortality in Elderly Patients With Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Ziprasidone is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning,Warnings and Precautions (5.2)]
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients With Dementia-Related Psychosis
In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. Ziprasidone is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.1)].
5.3 QT Prolongation and Risk of Sudden Death
Ziprasidone use should be avoided in combination with other drugs that are known to prolong the QTc interval [see Contraindications (4.1)and Drug Interactions (7.4)]. Additionally, clinicians should be alert to the identification of other drugs that have been consistently observed to prolong the QTc interval. Such drugs should not be prescribed with ziprasidone. Ziprasidone should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias [see Contraindications (4)].
A study directly comparing the QT/QTc prolonging effect of oral ziprasidone with several other drugs effective in the treatment of schizophrenia was conducted in patient volunteers. In the first phase of the trial, ECGs were obtained at the time of maximum plasma concentration when the drug was administered alone. In the second phase of the trial, ECGs were obtained at the time of maximum plasma concentration while the drug was co-administered with an inhibitor of the CYP4503A4 metabolism of the drug.
In the first phase of the study, the mean change in QTc from baseline was calculated for each drug, using a sample-based correction that removes the effect of heart rate on the QT interval. The mean increase in QTc from baseline for ziprasidone ranged from approximately 9 to 14 msec greater than for four of the comparator drugs (risperidone, olanzapine, quetiapine, and haloperidol), but was approximately 14 msec less than the prolongation observed for thioridazine.
In the second phase of the study, the effect of ziprasidone on QTc length was not augmented by the presence of a metabolic inhibitor (ketoconazole 200 mg twice daily).
In placebo-controlled trials in adults, oral ziprasidone increased the QTc interval compared to placebo by approximately 10 msec at the highest recommended daily dose of 160 mg. In clinical trials with oral ziprasidone, the electrocardiograms of 2/2988 (0.06%) patients who received ziprasidone and 1/440 (0.23%) patients who received placebo revealed QTc intervals exceeding the potentially clinically relevant threshold of 500 msec. In the ziprasidone-treated patients, neither case suggested a role of ziprasidone. One patient had a history of prolonged QTc and a screening measurement of 489 msec; QTc was 503 msec during ziprasidone treatment. The other patient had a QTc of 391 msec at the end of treatment with ziprasidone and upon switching to thioridazine experienced QTc measurements of 518 and 593 msec.
Some drugs that prolong the QT/QTc interval have been associated with the occurrence of torsade de pointes and with sudden unexplained death. The relationship of QT prolongation to torsade de pointes is clearest for larger increases (20 msec and greater) but it is possible that smaller QT/QTc prolongations may also increase risk, or increase it in susceptible individuals. Although torsade de pointes has not been observed in association with the use of ziprasidone in premarketing studies and experience is too limited to rule out an increased risk, there have been rare post-marketing reports (in the presence of multiple confounding factors) [see Adverse Reactions (6.2)].
As with other antipsychotic drugs and placebo, sudden unexplained deaths have been reported in patients taking ziprasidone at recommended doses. The premarketing experience for ziprasidone did not reveal an excess risk of mortality for ziprasidone compared to other antipsychotic drugs or placebo, but the extent of exposure was limited, especially for the drugs used as active controls and placebo. Nevertheless, ziprasidone’s larger prolongation of QTc length compared to several other antipsychotic drugs raises the possibility that the risk of sudden death may be greater for ziprasidone than for other available drugs for treating schizophrenia. This possibility needs to be considered in deciding among alternative drug products [see Indications and Usage (1)].
Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
It is recommended that patients being considered for ziprasidone treatment who are at risk for significant electrolyte disturbances, hypokalemia in particular, have baseline serum potassium and magnesium measurements. Hypokalemia (and/or hypomagnesemia) may increase the risk of QT prolongation and arrhythmia. Hypokalemia may result from diuretic therapy, diarrhea, and other causes. Patients with low serum potassium and/or magnesium should be repleted with those electrolytes before proceeding with treatment. It is essential to periodically monitor serum electrolytes in patients for whom diuretic therapy is introduced during ziprasidone treatment. Persistently prolonged QTc intervals may also increase the risk of further prolongation and arrhythmia, but it is not clear that routine screening ECG measures are effective in detecting such patients. Rather, ziprasidone should be avoided in patients with histories of significant cardiovascular illness, e.g., QT prolongation, recent acute myocardial infarction, uncompensated heart failure, or cardiac arrhythmia. Ziprasidone should be discontinued in patients who are found to have persistent QTc measurements >500 msec.
For patients taking ziprasidone who experience symptoms that could indicate the occurrence of torsade de pointes, e.g., dizziness, palpitations, or syncope, the prescriber should initiate further evaluation, e.g., Holter monitoring may be useful.
5.4 Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to exclude cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include: (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
5.5 Severe Cutaneous Adverse Reactions
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported with Ziprasidone exposure. DRESS consists of a combination of three or more of the following: cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, lymphadenopathy and one or more systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and pericarditis. DRESS is sometimes fatal. Discontinue ziprasidone if DRESS is suspected.
Other severe cutaneous adverse reactions
Other severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, have been reported with ziprasidone exposure. Severe cutaneous adverse reactions are sometimes fatal. Discontinue ziprasidone if severe cutaneous adverse reactions are suspected.
5.6 Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients undergoing treatment with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, ziprasidone should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on ziprasidone, drug discontinuation should be considered. However, some patients may require treatment with ziprasidone despite the presence of the syndrome.
5.7 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics. There have been few reports of hyperglycemia or diabetes in patients treated with ziprasidone. Although fewer patients have been treated with ziprasidone, it is not known if this more limited experience is the sole reason for the paucity of such reports. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. Precise risk estimates for hyperglycemia-related adverse reactions in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of antidiabetic treatment despite discontinuation of the suspect drug.
Pooled data from short-term, placebo-controlled studies in schizophrenia and bipolar disorder are presented in Tables 1-4. Note that for the flexible dose studies in both schizophrenia and bipolar disorder, each subject is categorized as having received either low (20 to 40 mg BID) or high (60 to 80 mg BID) dose based on the subject’s modal daily dose. In the tables showing categorical changes, the percentages (% column) are calculated as 100x (n/N).
Table 1: Glucose* Mean Change from Baseline in Short-Term (up to 6 weeks), Placebo-Controlled, Fixed-Dose, Oral Ziprasidone, Monotherapy Trials in Adult Patients With Schizophrenia
Mean Random Glucose Change from Baseline mg/dL (N) Ziprasidone Placebo 5 mg BID 20 mg BID 40 mg BID 60 mg BID 80 mg BID 100 mg BID -1.1 (N=45) +2.4 (N=179) -0.2 (N=146) -0.5 (N=119) -1.7 (N=104) +4.1 (N=85) +1.4 (N=260) *‟Random” glucose measurements—fasting/non-fasting status unknown
Table 2: Glucose* Categorical Changes in Short-Term (up to 6 weeks), Placebo-Controlled, Fixed-Dose, Oral Ziprasidone, Monotherapy Trials in Adult Patients With Schizophrenia
Laboratory Analyte Category Change (at least once) from Baseline Treatment Arm N n (%) Random Glucose Normal to High (<100 mg/dL to ≥126 mg/dL) Ziprasidone 438 77 (17.6%) Placebo 169 26 (15.4%) Borderline to High (≥100 mg/dL and <126 mg/dL to ≥126 mg/dL) Ziprasidone 159 54 (34%) Placebo 66 22 (33.3%) *‟Random” glucose measurements – fasting/non-fasting status unknown
In long-term (at least 1 year), placebo-controlled, flexible-dose studies in schizophrenia, the mean change from baseline in random glucose for ziprasidone 20 to 40 mg BID was -3.4 mg/dL (N=122); for ziprasidone 60 to 80 mg BID was +1.3 mg/dL (N=10); and for placebo was +0.3 mg/dL (N=71).
Table 3: Glucose* Mean Change from Baseline in Short-Term (up to 6 weeks), Placebo-Controlled, Flexible-Dose, Oral Ziprasidone, Monotherapy Trials in Adult Patients With Bipolar Disorder
Mean Fasting Glucose Change from Baseline mg/dL (N) Ziprasidone Placebo Low Dose: 20 to 40 mg BID High Dose: 60 to 80 mg BID +0.1 (N=206) +1.6 (N=166) +1.4 (N=287) *Fasting
Table 4: Glucose* Categorical Changes in Short-Term (up to 6 weeks), Placebo-Controlled, Flexible-Dose, Oral Ziprasidone, Monotherapy Trials in Adult Patients With Bipolar Disorder
Laboratory Analyte Category Change (at least once) from Baseline Treatment Arm N n (%) Fasting Glucose Normal to High (<100 mg/dL to ≥126 mg/dL) Ziprasidone 272 5 (1.8%) Placebo 210 2 (1%) Borderline to High (≥100 mg/dL and <126 mg/dL to ≥126 mg/dL) Ziprasidone 79 12 (15.2%) Placebo 71 7 (9.9%) *Fasting
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics. Pooled data from short-term, placebo-controlled studies in schizophrenia and bipolar disorder are presented in Tables 5-8.
Table 5: Lipid* Mean Change from Baseline in Short-Term (up to 6 weeks), Placebo-Controlled, Fixed-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Schizophrenia
Mean Lipid Change from Baseline mg/dL (N) Laboratory Analyte Ziprasidone Placebo 5 mg BID 20 mg BID 40 mg BID 60 mg BID 80 mg BID 100 mg BID Triglycerides -12.9 (N=45) -9.6 (N=181) -17.3 (N=146) -0.05 (N=120) -16 (N=104) +0.8 (N=85) -18.6 (N=260) Total Cholesterol -3.6 (N=45) -4.4 (N=181) -8.2 (N=147) -3.6 (N=120) -10 (N=104) -3.6 (N=85) -4.7 (N=261)
*‟Random” lipid measurements, fasting/non-fasting status unknown
Table 6: Lipid* Categorical Changes in Short-Term (up to 6 weeks), Placebo-Controlled, Fixed-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Schizophrenia
Laboratory Analyte Category Change (at least once) from Baseline Treatment Arm N n (%) Triglycerides Increase by ≥50 mg/dL Ziprasidone 681 232 (34.1%) Placebo 260 53 (20.4%) Normal to High (<150 mg/dL to ≥200 mg/dL) Ziprasidone 429 63 (14.7%) Placebo 152 12 (7.9%) Borderline to High (≥150 mg/dL and <200 mg/dL to ≥200 mg/dL) Ziprasidone 92 43 (46.7%) Placebo 41 12 (29.3%) Total Cholesterol Increase by ≥40 mg/dL Ziprasidone 682 76 (11.1%) Placebo 261 26 (10%) Normal to High (<200 mg/dL to ≥240 mg/dL) Ziprasidone 380 15 (3.9%) Placebo 145 0 (0%) Borderline to High (≥200 mg/dL and <240 mg/dL to ≥240 mg/dL) Ziprasidone 207 56 (27.1%) Placebo 82 22 (26.8%) *‟Random” lipid measurements, fasting/non-fasting status unknown
In long-term (at least 1 year), placebo-controlled, flexible-dose studies in schizophrenia, the mean change from baseline in random triglycerides for ziprasidone 20 to 40 mg BID was +26.3 mg/dL (N=15); for ziprasidone 60 to 80 mg BID was -39.3 mg/dL (N=10); and for placebo was +12.9 mg/dL (N=9). In long-term (at least 1 year), placebo-controlled, flexible-dose studies in schizophrenia, the mean change from baseline in random total cholesterol for ziprasidone 20 to 40 mg BID was +2.5 mg/dL (N=14); for ziprasidone 60 to 80 mg BID was -19.7 mg/dL (N=10); and for placebo was -28 mg/dL (N=9).
Table 7: Lipid* Mean Change from Baseline in Short-Term (up to 6 weeks), Placebo-Controlled, Flexible-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Bipolar Disorder
Laboratory Analyte Mean Change from Baseline mg/dL (N) Ziprasidone Placebo Low Dose:20 to 40 mg BID High Dose:60 to 80 mg BID Fasting Triglycerides +0.95 (N=206) -3.5 (N=165) +8.6 (N=286) Fasting Total Cholesterol -2.8 (N=206) -3.4 (N=165) -1.6 (N=286) Fasting LDL Cholesterol -3 (N=201) -3.1 (N=158) -1.97 (N=270) Fasting HDL cholesterol -0.09 (N=206) +0.3 (N=165) -0.9 (N=286) *Fasting
Table 8: Lipid* Categorical Changes in Short-Term (up to 6 weeks), Placebo-Controlled, Flexible-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Bipolar Disorder
Laboratory Analyte Category Change (at least once) from Baseline Treatment Arm N n (%) Fasting Triglycerides Increase by ≥50 mg/dL Ziprasidone 371 66 (17.8%) Placebo 286 62 (21.7%) Normal to High (<150 mg/dL to ≥200 mg/dL) Ziprasidone 225 15 (6.7%) Placebo 179 13 (7.3%) Borderline to High (≥150 mg/dL and <200 mg/dL to ≥200 mg/dL) Ziprasidone 58 16 (27.6%) Placebo 47 14 (29.8%) Fasting Total Cholesterol Increase by ≥40 mg/dL Ziprasidone 371 30 (8.1%) Placebo 286 13 (4.5%) Normal to High (<200 mg/dL to ≥240 mg/dL) Ziprasidone 204 5 (2.5%) Placebo 151 2 (1.3%) Borderline to High (≥200 mg/dL and <240 mg/dL to ≥240 mg/dL) Ziprasidone 106 10 (9.4%) Placebo 87 15 (17.2%) Fasting LDL Cholesterol Increase by ≥30 mg/dL Ziprasidone 359 39 (10.9%) Placebo 270 17 (6.3%) Normal to High (<100 mg/dL to ≥160 mg/dL) Ziprasidone 115 0 (0%) Placebo 89 1 (1.1%) Borderline to High (≥100 mg/dL and <160 mg/dL to ≥160 mg/dL) Ziprasidone 193 18 (9.3%) Placebo 141 14 (9.9%) Fasting HDL Normal (>=40 mg/dL) to Low (<40 mg/dL) Ziprasidone 283 22 (7.8%) Placebo 220 24 (10.9%) *Fasting
Weight Gain
Weight gain has been observed with atypical antipsychotic use. Monitoring of weight is recommended. Pooled data from short-term, placebo-controlled studies in schizophrenia and bipolar disorder are presented in Tables 9-10.
Table 9: Weight Mean Changes in Short-Term (up to 6 weeks), Placebo-Controlled, Fixed-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Schizophrenia
Ziprasidone Placebo 5 mg BID 20 mg BID 40 mg BID 60 mg BID 80 mg BID 100 mg BID Mean Weight (kg) Changes from Baseline (N) +0.3 (N=40) +1 (N=167) +1(N=135) +0.7 (N=109) +1.1 (N=97) +0.9 (N=74) -0.4 (227) Proportion of Patients With ≥7% Increase in Weight from Baseline (N) 0% (N=40) 9% (N=167) 10.4% (N=135) 7.3% (N=109) 15.5% (N=97) 10.8% (N=74) 4% (N=227) In long-term (at least 1 year), placebo-controlled, flexible-dose studies in schizophrenia, the mean change from baseline weight for ziprasidone 20 to 40 mg BID was -2.3 kg (N=124); for ziprasidone 60 to 80 mg BID was +2.5 kg (N=10); and for placebo was -2.9 kg (N=72). In the same long-term studies, the proportion of subjects with ≥ 7% increase in weight from baseline for ziprasidone 20 to 40 mg BID was 5.6% (N=124); for ziprasidone 60-80 mg BID was 20% (N=10), and for placebo was 5.6% (N=72). In a long-term (at least 1 year), placebo-controlled, fixed-dose study in schizophrenia, the mean change from baseline weight for ziprasidone 20 mg BID was -2.6 kg (N=72); for ziprasidone 40 mg BID was -3.3 kg (N=69); for ziprasidone 80 mg BID was -2.8 kg (N=70) and for placebo was -3.8 kg (N=70). In the same long-term fixed-dose schizophrenia study, the proportion of subjects with ≥ 7% increase in weight from baseline for ziprasidone 20 mg BID was 5.6% (N=72); for ziprasidone 40 mg BID was 2.9% (N=69); for ziprasidone 80 mg BID was 5.7% (N=70) and for placebo was 2.9% (N=70).
Table 10: Summary of Weight Change in Short-Term (up to 6 weeks), Placebo-Controlled, Flexible-Dose, Oral Ziprasidone Monotherapy Trials in Adult Patients With Bipolar Disorder:
Ziprasidone Placebo Low Dose:
20 to 40 mg BIDHigh Dose*:60 to 80 mg BID Mean Weight (kg) Changes from Baseline (N) +0.4 (N=295) +0.4 (N=388) +0.1 (N=451) Proportion of Patients With ≥ 7% Increase in Weight from Baseline (N) 2.4% (N=295) 4.4% (N=388) 1.8% (N=451) * Note that in the High Dose group, there were 2 subjects with modal 200 mg total daily dose and 1 subject with modal 100 mg total daily dose.
Schizophrenia -The proportions of patients meeting a weight gain criterion of ≥7% of body weight were compared in a pool of four 4- and 6-week placebo-controlled schizophrenia clinical trials, revealing a statistically significantly greater incidence of weight gain for ziprasidone (10%) compared to placebo (4%). A median weight gain of 0.5 kg was observed in ziprasidone patients compared to no median weight change in placebo patients. In this set of clinical trials, weight gain was reported as an adverse reaction in 0.4% and 0.4% of ziprasidone and placebo patients, respectively. During long-term therapy with ziprasidone, a categorization of patients at baseline on the basis of body mass index (BMI) revealed the greatest mean weight gain and highest incidence of clinically significant weight gain (> 7% of body weight) in patients with low BMI (<23) compared to normal (23 to 27) or overweight patients (>27). There was a mean weight gain of 1.4 kg for those patients with a “low” baseline BMI, no mean change for patients with a “normal” BMI, and a 1.3 kg mean weight loss for patients who entered the program with a “high” BMI.
Bipolar Disorder – During a 6 month placebo-controlled bipolar maintenance study in adults with ziprasidone as an adjunct to lithium or valproate, the incidence of clinically significant weight gain (≥ 7% of body weight) during the double-blind period was 5.6% for both ziprasidone and placebo treatment groups who completed the 6 months of observation for relapse. Interpretation of these findings should take into consideration that only patients who adequately tolerated ziprasidone entered the double-blind phase of the study, and there were substantial dropouts during the open label phase.
5.8 Rash
In premarketing trials with ziprasidone, about 5% of patients developed rash and/or urticaria, with discontinuation of treatment in about one-sixth of these cases. The occurrence of rash was related to dose of ziprasidone, although the finding might also be explained by the longer exposure time in the higher dose patients. Several patients with rash had signs and symptoms of associated systemic illness, e.g., elevated WBCs. Most patients improved promptly with adjunctive treatment with antihistamines or steroids and/or upon discontinuation of ziprasidone, and all patients experiencing these reactions were reported to recover completely. Upon appearance of rash for which an alternative etiology cannot be identified, ziprasidone should be discontinued.
5.9 Orthostatic Hypotension
Ziprasidone may induce orthostatic hypotension associated with dizziness, tachycardia, and, in some patients, syncope, especially during the initial dose-titration period, probably reflecting its α1-adrenergic antagonist properties. Syncope was reported in 0.6% of the patients treated with ziprasidone.
Ziprasidone should be used with particular caution in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease, or conditions which would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications).
5.10 Falls
Antipsychotic drugs (which include ziprasidone) may cause somnolence, postural hypotension, and motor and sensory instability, which could lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.11 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents. Agranulocytosis (including fatal cases) has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue ziprasidone at the first sign of decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue ziprasidone and have their WBC followed until recovery.
5.12 Seizures
During clinical trials, seizures occurred in 0.4% of patients treated with ziprasidone. There were confounding factors that may have contributed to the occurrence of seizures in many of these cases. As with other antipsychotic drugs, ziprasidone should be used cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold, e.g., Alzheimer’s dementia. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
5.13 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer’s dementia. Ziprasidone and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia [see Boxed Warning].
5.14 Hyperprolactinemia
As with other drugs that antagonize dopamine D2 receptors, ziprasidone elevates prolactin levels in humans. Increased prolactin levels were also observed in animal studies with this compound, and were associated with an increase in mammary gland neoplasia in mice; a similar effect was not observed in rats [see Nonclinical Toxicology (13.1)]. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with previously detected breast cancer. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans; the available evidence is considered too limited to be conclusive at this time.
Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating compounds, the clinical significance of elevated serum prolactin levels is unknown for most patients. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density.
5.15 Potential for Cognitive and Motor Impairment
Somnolence was a commonly reported adverse reaction in patients treated with ziprasidone. In the 4- and 6-week placebo-controlled trials in adults, somnolence was reported in 14% of patients on ziprasidone compared to 7% of placebo patients. Somnolence led to discontinuation in 0.3% of the patients in short-term clinical trials in adults.
Since ziprasidone has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about performing activities requiring mental alertness, such as operating a motor vehicle (including automobiles) or operating hazardous machinery until they are reasonably certain that ziprasidone therapy does not affect them adversely.
5.16 Priapism
One case of priapism was reported in the premarketing database. While the relationship of the reaction to ziprasidone use has not been established, other drugs with alpha-adrenergic blocking effects have been reported to induce priapism, and it is possible that ziprasidone may share this capacity. Severe priapism may require surgical intervention.
5.17 Body Temperature Regulation
Although not reported with ziprasidone in premarketing trials, disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing ziprasidone for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
5.18 Suicide
The possibility of a suicide attempt is inherent in psychotic illness or bipolar disorder, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for ziprasidone should be written for the smallest quantity of capsules consistent with good patient management in order to reduce the risk of overdose.
5.19 Patients With Concomitant Illnesses
Clinical experience with ziprasidone in patients with certain concomitant systemic illnesses is limited [see Usein Specific Populations (8.6),(8.7)]
Ziprasidone has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were excluded from premarketing clinical studies. Because of the risk of QTc prolongation and orthostatic hypotension with ziprasidone, caution should be observed in cardiac patients [see Warnings and Precautions (5.3), (5.9)]
Close5.20 Laboratory Tests
Patients being considered for ziprasidone treatment that are at risk of significant electrolyte disturbances should have baseline serum potassium and magnesium measurements. Low serum potassium and magnesium should be replaced before proceeding with treatment. Patients who are started on diuretics during Ziprasidone therapy need periodic monitoring of serum potassium and magnesium. Ziprasidone should be discontinued in patients who are found to have persistent QTc measurements >500 msec. [see Warningsand Precautions (5.3) ]
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical trials in adults for oral ziprasidone included approximately 5700 patients and/or normal subjects exposed to one or more doses of ziprasidone. Of these 5700, over 4800 were patients who participated in multiple-dose effectiveness trials, and their experience corresponded to approximately 1831 patient-years. These patients include: (1) 4331 patients who participated in multiple-dose trials, predominantly in schizophrenia, representing approximately 1698 patient-years of exposure as of February 5, 2000; and (2) 472 patients who participated in bipolar mania trials representing approximately 133 patient-years of exposure. An additional 127 patients with bipolar disorder participated in a long-term maintenance treatment study representing approximately 74.7 patient-years of exposure to ziprasidone. The conditions and duration of treatment with ziprasidone included open-label and double-blind studies, inpatient and outpatient studies and short-term and longer-term exposure.
Adverse reactions during exposure were obtained by collecting voluntarily reported adverse experiences, as well as results of physical examinations, vital signs, weights, laboratory analyses, ECGs, and results of ophthalmologic examinations.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse reaction of the type listed. A reaction was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials with Oral Ziprasidone
The following findings are based on the short-term placebo-controlled premarketing trials for schizophrenia (a pool of two 6-week, and two 4-week fixed-dose trials) and bipolar mania (a pool of two 3-week flexible-dose trials) in which ziprasidone was administered in doses ranging from 10 to 200 mg/day.
Commonly Observed Adverse Reactions in Short Term-Placebo-Controlled Trials
The following adverse reactions were the most commonly observed adverse reactions associated with the use of ziprasidone (incidence of 5% or greater) and not observed at an equivalent incidence among placebo-treated patients (ziprasidone incidence at least twice that for placebo):
Schizophrenia trials (see Table 11)
• Somnolence
• Respiratory Tract Infection
Bipolar trials (see Table 12)
• Somnolence
• Extrapyramidal Symptoms which includes the following adverse reaction terms: extrapyramidal syndrome, hypertonia, dystonia, dyskinesia, hypokinesia, tremor, paralysis and twitching. None of these adverse reactions occurred individually at an incidence greater than 10% in bipolar mania trials.
• Dizziness which includes the adverse reaction terms dizziness and lightheadedness.
• Akathisia
• Abnormal Vision
• Asthenia
• Vomiting
SCHIZOPHRENIA
Adverse Reactions Associated With Discontinuation of Treatment in Short-Term, Placebo-Controlled Trials of Oral Ziprasidone Approximately 4.1% (29/702) of ziprasidone-treated patients in short-term, placebo-controlled studies discontinued treatment due to an adverse reaction, compared with about 2.2% (6/273) on placebo. The most common reaction associated with dropout was rash, including 7 dropouts for rash among ziprasidone patients (1%) compared to no placebo patients [see Warnings and Precautions (5.8)].
Adverse Reactions Occurring at an Incidence of 2% or More Among Ziprasidone-Treated Patients in Short-Term, Oral, Placebo-Controlled Trials
Table 11 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse reactions that occurred during acute therapy (up to 6 weeks) in predominantly patients with schizophrenia, including only those reactions that occurred in 2% or more of patients treated with ziprasidone and for which the incidence in patients treated with ziprasidone was greater than the incidence in placebo-treated patients.
Table 11: Treatment-Emergent Adverse Reaction Incidence In Short-Term Oral Placebo-Controlled Trials – Schizophrenia
Percentage of Patients Reporting Reaction Body System/Adverse Reaction Ziprasidone (N=702) Placebo (N=273) Body as a Whole Asthenia 5 3 Accidental Injury 4 2 Chest Pain 3 2 Cardiovascular Tachycardia 2 1 Digestive Nausea 10 7 Constipation 9 8 Dyspepsia 8 7 Diarrhea 5 4 Dry Mouth 4 2 Anorexia 2 1 Nervous Extrapyramidal Symptoms* 14 8 Somnolence 14 7 Akathisia 8 7 Dizziness** 8 6 Respiratory Respiratory Tract Infection 8 3 Rhinitis 4 2 Cough Increased 3 1 Skin and Appendages Rash 4 3 Fungal Dermatitis 2 1 Special Senses Abnormal Vision 3 2 * Extrapyramidal Symptoms includes the following adverse reaction terms: extrapyramidal syndrome, hypertonia, dystonia, dyskinesia, hypokinesia, tremor, paralysis and twitching. None of these adverse reactions occurred individually at an incidence greater than 5% in schizophrenia trials.
** Dizziness includes the adverse reaction terms dizziness and lightheadedness.
Dose Dependency of Adverse Reactions in Short-Term, Fixed-Dose, Placebo-Controlled Trials An analysis for dose response in the schizophrenia 4-study pool revealed an apparent relation of adverse reaction to dose for the following reactions: asthenia, postural hypotension, anorexia, dry mouth, increased salivation, arthralgia, anxiety, dizziness, dystonia, hypertonia, somnolence, tremor, rhinitis, rash, and abnormal vision.
Extrapyramidal Symptoms (EPS) - The incidence of reported EPS (which included the adverse reaction terms extrapyramidal syndrome, hypertonia, dystonia, dyskinesia, hypokinesia, tremor, paralysis and twitching) for ziprasidone-treated patients in the short-term, placebo-controlled schizophrenia trials was 14% vs. 8% for placebo. Objectively collected data from those trials on the Simpson-Angus Rating Scale (for EPS) and the Barnes Akathisia Scale (for akathisia) did not generally show a difference between ziprasidone and placebo.
Dystonia - Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Vital Sign Changes - Ziprasidone is associated with orthostatic hypotension [see Warnings and Precautions (5.9)]
ECG Changes - Ziprasidone is associated with an increase in the QTc interval [see Warnings and Precautions (5.3)]. In the schizophrenia trials, ziprasidone was associated with a mean increase in heart rate of 1.4 beats per minute compared to a 0.2 beats per minute decrease among placebo patients.
Other Adverse Reactions Observed During the Premarketing Evaluation of Oral Ziprasidone
Following is a list of COSTART terms that reflect treatment-emergent adverse reactions as defined in the introduction to the ADVERSE REACTIONSsection reported by patients treated with ziprasidone in schizophrenia trials at multiple doses >4 mg/day within the database of 3834 patients. All reported reactions are included except those already listed in Table 11 or elsewhere in labeling, those reaction terms that were so general as to be uninformative, reactions reported only once and that did not have a substantial probability of being acutely life-threatening, reactions that are part of the illness being treated or are otherwise common as background reactions, and reactions considered unlikely to be drug-related. It is important to emphasize that, although the reactions reported occurred during treatment with ziprasidone, they were not necessarily caused by it.
Adverse reactions are further categorized by body system and listed in order of decreasing frequency according to the following definitions:
Frequent -adverse reactions occurring in at least 1/100 patients (≥1% of patients) (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing);
Infrequent -adverse reactions occurring in 1/100 to 1/1000 patients (in 0.1 to 1% of patients)
Rare – adverse reactions occurring in fewer than 1/1000 patients (<0.1% of patients).
Body as a Whole
Frequent abdominal pain, flu syndrome, fever, accidental fall, face edema, chills, photosensitivity reaction, flank pain, hypothermia, motor vehicle accident
Cardiovascular System
Frequent tachycardia, hypertension, postural hypotension
Infrequent bradycardia, angina pectoris, atrial fibrillation
Rare first degree AV block, bundle branch block, phlebitis, pulmonary embolus, cardiomegaly, cerebral infarct, cerebrovascular accident, deep thrombophlebitis, myocarditis, thrombophlebitis
Digestive System
Frequent anorexia, vomiting
Infrequent rectal hemorrhage, dysphagia, tongue edema
Rare gum hemorrhage, jaundice, fecal impaction, gamma glutamyl transpeptidase increased, hematemesis, cholestatic jaundice, hepatitis, hepatomegaly, leukoplakia of mouth, fatty liver deposit, melena
Endocrine
Rare hypothyroidism, hyperthyroidism, thyroiditis
Hemic and Lymphatic System
Infrequent anemia, ecchymosis, leukocytosis, leukopenia, eosinophilia, lymphadenopathy
Rare thrombocytopenia, hypochromic anemia, lymphocytosis, monocytosis, basophilia, lymphedema, polycythemia, thrombocythemia
Metabolic and Nutritional Disorders
Infrequent thirst, transaminase increased, peripheral edema, hyperglycemia, creatine phosphokinase increased, alkaline phosphatase increased, hypercholesteremia, dehydration, lactic dehydrogenase increased, albuminuria, hypokalemia
Rare BUN increased, creatinine increased, hyperlipemia, hypocholesteremia, hyperkalemia, hypochloremia, hypoglycemia, hyponatremia, hypoproteinemia, glucose tolerance decreased, gout, hyperchloremia, hyperuricemia, hypocalcemia, hypoglycemicreaction, hypomagnesemia, ketosis, respiratory alkalosis
Musculoskeletal System
Frequent myalgia
Infrequent tenosynovitis
Rare myopathy
Nervous System
Frequent agitation, extrapyramidal syndrome, tremor, dystonia, hypertonia, dyskinesia, hostility, twitching, paresthesia, confusion, vertigo, hypokinesia, hyperkinesia, abnormal gait, oculogyric crisis, hypesthesia, ataxia, amnesia, cogwheel rigidity, delirium, hypotonia, akinesia, dysarthria, withdrawal syndrome, buccoglossal syndrome, choreoathetosis, diplopia, incoordination, neuropathy
Infrequent paralysis
Rare myoclonus, nystagmus, torticollis, circumoral paresthesia, opisthotonos, reflexes increased, trismus
Respiratory System
Frequent dyspnea
Infrequent pneumonia, epistaxis
Rare hemoptysis, laryngismus
Skin and Appendages
Infrequent maculopapular rash, urticaria, alopecia, eczema, exfoliative dermatitis, contact dermatitis, vesiculobullous rash
Special Senses
Frequent fungal dermatitis
Infrequent conjunctivitis, dry eyes, tinnitus, blepharitis, cataract, photophobia
Rare eye hemorrhage, visual field defect, keratitis, keratoconjunctivitis
Urogenital System
Infrequent impotence, abnormal ejaculation, amenorrhea, hematuria, menorrhagia, female lactation, polyuria, urinary retention metrorrhagia, male sexual dysfunction, anorgasmia, glycosuria
Rare gynecomastia, vaginal hemorrhage, nocturia, oliguria, female sexual dysfunction, uterine hemorrhage
BIPOLAR DISORDER
Acute Treatment of Manic or Mixed Episodes in Adults
Adverse Reactions Associated With Discontinuation of Treatment in Short Term, Placebo-Controlled Trials Approximately 6.5% (18/279) of ziprasidone-treated patients in short-term, placebo-controlled studies discontinued treatment due to an adverse reaction, compared with about 3.7% (5/136) on placebo. The most common reactions associated with dropout in the ziprasidone-treated patients were akathisia, anxiety, depression, dizziness, dystonia, rash and vomiting, with 2 dropouts for each of these reactions among ziprasidone patients (1%) compared to one placebo patient each for dystonia and rash (1%) and no placebo patients for the remaining adverse reactions.
Adverse Reactions Occurring at an Incidence of 2% or More Among Ziprasidone-Treated Patients in Short-Term, Oral, Placebo-Controlled Trials
Table 12 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse reactions that occurred during acute therapy (up to 3 weeks) in patients with bipolar mania, including only those reactions that occurred in 2% or more of patients treated with ziprasidone and for which the incidence in patients treated with ziprasidone was greater than the incidence in placebo-treated patients.
Table 12: Treatment-Emergent Adverse Reactions Incidence In Short-Term Oral Placebo-Controlled Trials – Manic and Mixed Episodes Associated With Bipolar Disorder
Percentage of Patients Reporting Reaction Body System/Adverse Reaction Ziprasidone
(N=279)Placebo
(N=136)Body as a Whole Headache 18 17 Asthenia 6 2 Accidental Injury 4 1 Cardiovascular Hypertension 3 2 Digestive Nausea 10 7 Diarrhea 5 4 Dry Mouth 5 4 Vomiting 5 2 Increased Salivation 4 0 Tongue Edema 3 1 Dysphagia 2 0 Musculoskeletal Myalgia 2 0 Nervous Somnolence 31 12 Extrapyramidal Symptoms* 31 12 Dizziness** 16 7 Akathisia 10 5 Anxiety 5 4 Hypesthesia 2 1 Speech Disorder 2 0 Respiratory Pharyngitis 3 1 Dyspnea 2 1 Skin and Appendages Fungal Dermatitis 2 1 Special Senses Abnormal Vision 6 3 * Extrapyramidal Symptoms includes the following adverse reaction terms: extrapyramidal syndrome, hypertonia, dystonia, dyskinesia, hypokinesia, tremor, paralysis and twitching. None of these adverse reactions occurred individually at an incidence greater than 10% in bipolar mania trials.
** Dizziness includes the adverse reaction terms dizziness and lightheadedness.
Explorations for interactions on the basis of gender did not reveal any clinically meaningful differences in the adverse reaction occurrence on the basis of this demographic factor.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ziprasidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reaction reports not listed above that have been received since market introduction include rare occurrences of the following : Cardiac Disorders: Tachycardia, torsade de pointes (in the presence of multiple confounding factors), [see Warnings and Precautions (5.3)]; Digestive System Disorders: Swollen Tongue; Reproductive System and Breast Disorders: Galactorrhea, priapism; Nervous System Disorders: Facial Droop, neuroleptic malignant syndrome, serotonin syndrome (alone or in combination with serotonergic medicinal products), tardive dyskinesia; Psychiatric Disorders: Insomnia, mania/hypomania; Skin and subcutaneous Tissue Disorders: Allergic reaction (such as allergic dermatitis, angioedema, orofacial edema, urticaria), rash, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS); Urogenital System Disorders: Enuresis, urinary incontinence; Vascular Disorders: Postural hypotension, syncope.
-
7 DRUG INTERACTIONSDrug-drug interactions can be pharmacodynamic (combined pharmacologic effects) or pharmacokinetic (alteration of plasma levels). The risks of using ziprasidone in combination with other drugs have ...
Drug-drug interactions can be pharmacodynamic (combined pharmacologic effects) or pharmacokinetic (alteration of plasma levels). The risks of using ziprasidone in combination with other drugs have been evaluated as described below. All interactions studies have been conducted with oral ziprasidone. Based upon the pharmacodynamic and pharmacokinetic profile of ziprasidone, possible interactions could be anticipated:
7.1 Metabolic Pathway
Approximately two-thirds of ziprasidone is metabolized via a combination of chemical reduction by glutathione and enzymatic reduction by aldehyde oxidase. There are no known clinically relevant inhibitors or inducers of aldehyde oxidase. Less than one-third of ziprasidone metabolic clearance is mediated by cytochrome P450 catalyzed oxidation.
7.2 In Vitro Studies
An in vitro enzyme inhibition study utilizing human liver microsomes showed that ziprasidone had little inhibitory effect on CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, and thus would not likely interfere with the metabolism of drugs primarily metabolized by these enzymes. There is little potential for drug interactions with ziprasidone due to displacement [see Clinical Pharmacology (12.3)].
7.3 Pharmacodynamic Interactions
Ziprasidone should not be used with any drug that prolongs the QT interval [see Contraindications (4.1)].
Given the primary CNS effects of ziprasidone, caution should be used when it is taken in combination with other centrally acting drugs.
Because of its potential for inducing hypotension, ziprasidone may enhance the effects of certain antihypertensive agents.
Ziprasidone may antagonize the effects of levodopa and dopamine agonists.
7.4 Pharmacokinetic Interactions
Carbamazepine
Carbamazepine is an inducer of CYP3A4; administration of 200 mg twice daily for 21 days resulted in a decrease of approximately 35% in the AUC of ziprasidone. This effect may be greater when higher doses of carbamazepine are administered.
Ketoconazole
Ketoconazole, a potent inhibitor of CYP3A4, at a dose of 400 mg QD for 5 days, increased the AUC and Cmax of ziprasidone by about 35 to 40%. Other inhibitors of CYP3A4 would be expected to have similar effects.
Cimetidine
Cimetidine at a dose of 800 mg QD for 2 days did not affect ziprasidone pharmacokinetics.
Antacid
The co-administration of 30 mL of Maalox® with ziprasidone did not affect the pharmacokinetics of ziprasidone.
7.5 Lithium
Ziprasidone at a dose of 40 mg twice daily administered concomitantly with lithium at a dose of 450 mg twice daily for 7 days did not affect the steady-state level or renal clearance of lithium. Ziprasidone dosed adjunctively to lithium in a maintenance trial of bipolar patients did not affect mean therapeutic lithium levels.
7.6 Oral Contraceptives
In vivo studies have revealed no effect of ziprasidone on the pharmacokinetics of estrogen or progesterone components. Ziprasidone at a dose of 20 mg twice daily did not affect the pharmacokinetics of concomitantly administered oral contraceptives, ethinyl estradiol (0.03 mg) and levonorgestrel (0.15 mg).
7.7 Dextromethorphan
Consistent with in vitro results, a study in normal healthy volunteers showed that ziprasidone did not alter the metabolism of dextromethorphan, a CYP2D6 model substrate, to its major metabolite, dextrorphan. There was no statistically significant change in the urinary dextromethorphan/dextrorphan ratio.
7.8 Valproate
A pharmacokinetic interaction of ziprasidone with valproate is unlikely due to the lack of common metabolic pathways for the two drugs. Ziprasidone dosed adjunctively to valproate in a maintenance trial of bipolar patients did not affect mean therapeutic valproate levels.
7.9 Other Concomitant Drug Therapy
Population pharmacokinetic analysis of schizophrenic patients enrolled in controlled clinical trials has not revealed evidence of any clinically significant pharmacokinetic interactions with benztropine, propranolol, or lorazepam.
Close7.10 Food Interaction
The absolute bioavailability of a 20 mg dose under fed conditions is approximately 60%. The absorption of ziprasidone is increased up to two-fold in the presence of food [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including ziprasidone capsules ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including ziprasidone capsules, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs, including ziprasidone capsules, during the third trimester are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall available data from published epidemiologic studies of pregnant women exposed to ziprasidone have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia or bipolar I disorder and with exposure to antipsychotics, including ziprasidone capsules, during pregnancy (see Clinical Considerations).
In animal studies, ziprasidone administration to pregnant rats and rabbits during organogenesis caused developmental toxicity at doses similar to recommended human doses, and was teratogenic in rabbits at 3 times the maximum recommended human dose (MRHD). Rats exposed to ziprasidone during gestation and lactation exhibited increased perinatal pup mortality and delayed neurobehavioral and functional development of offspring at doses less than or similar to human therapeutic doses (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
There is risk to the mother from untreated schizophrenia or bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Schizophrenia and bipolar I disorder are associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/neonatal adverse reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs, including ziprasidone capsules, during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A retrospective cohort study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects.
Animal Data
When ziprasidone was administered to pregnant rabbits during the period of organogenesis, an increased incidence of fetal structural abnormalities (ventricular septal defects and other cardiovascular malformations, and kidney alterations) was observed at a dose of 30 mg/kg/day (3 times the MRHD of 200 mg/day based on mg/m2 body surface area). There was no evidence to suggest that these developmental effects were secondary to maternal toxicity. The developmental no effect dose was 10 mg/kg/day (equivalent to the MRHD based on a mg/m2 body surface area). In rats, embryofetal toxicity (decreased fetal weights, delayed skeletal ossification) was observed following administration of 10 to 160 mg/kg/day (0.5 to 8 times the MRHD based on mg/m2 body surface area) during organogenesis or throughout gestation, but there was no evidence of teratogenicity. Doses of 40 and 160 mg/kg/day (2 and 8 times the MRHD based on mg/m2 body surface area) were associated with maternal toxicity. The developmental no-effect dose is 5 mg/kg/day (0.2 times the MRHD based on mg/m2 body surface area).
There was an increase in the number of pups born dead and a decrease in postnatal survival through the first 4 days of lactation among the offspring of female rats treated during gestation and lactation with doses of 10 mg/kg/day (0.5 times the MRHD based on mg/m2 body surface area) or greater. Offspring developmental delays (decreased pup weights) and neurobehavioral functional impairment (eye opening air righting) were observed at doses of 5 mg/kg/day (0.2 times the MRHD based on mg/m2 body surface area) or greater. A no-effect level was not established for these effects.
8.2 Lactation
Risk Summary
Limited data from a published case report indicate the presence of ziprasidone in human milk. Although there are no reports of adverse effects on a breastfed infant exposed to ziprasidone via breast milk, there are reports of excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements) in infants exposed to other atypical antipsychotics through breast milk (see Clinical Considerations). There is no information on the effects of ziprasidone on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ziprasidone capsules and any potential adverse effects on the breastfed child from ziprasidone capsules or from the mother’s underlying condition.
Clinical Considerations
Infants exposed to ziprasidone capsules should be monitored for excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements).
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the pharmacologic action of ziprasidone (D2 antagonism), treatment with ziprasidone capsules may result in an increase in serum prolactin levels, which may lead to a reversible reduction in fertility in females of reproductive potential [see Warnings and Precautions (5.15) and Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of ziprasidone have not been established in pediatric patients.
Pediatric information is approved for Viatris Specialty LLC’s Geodon (ziprasidone HCl) capsules. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of ziprasidone, 2.4 percent were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Nevertheless, the presence of multiple factors that might increase the pharmacodynamic response to ziprasidone, or cause poorer tolerance or orthostasis, should lead to consideration of a lower starting dose, slower titration, and careful monitoring during the initial dosing period for some elderly patients.
8.6 Renal Impairment
Because ziprasidone is highly metabolized, with less than 1% of the drug excreted unchanged, renal impairment alone is unlikely to have a major impact on the pharmacokinetics of ziprasidone. The pharmacokinetics of ziprasidone following 8 days of 20 mg twice daily dosing were similar among subjects with varying degrees of renal impairment (n=27), and subjects with normal renal function, indicating that dosage adjustment based upon the degree of renal impairment is not required. Ziprasidone is not removed by hemodialysis.
8.7 Hepatic Impairment
As ziprasidone is cleared substantially by the liver, the presence of hepatic impairment would be expected to increase the AUC of ziprasidone; a multiple-dose study at 20 mg twice daily for 5 days in subjects (n=13) with clinically significant (Childs-Pugh Class A and B) cirrhosis revealed an increase in AUC 0-12 of 13% and 34% in Childs-Pugh Class A and B, respectively, compared to a matched control group (n=14). A half-life of 7.1 hours was observed in subjects with cirrhosis compared to 4.8 hours in the control group.
8.8 Age and Gender Effects
In a multiple-dose (8 days of treatment) study involving 32 subjects, there was no difference in the pharmacokinetics of ziprasidone between men and women or between elderly (>65 years) and young (18 to 45 years) subjects. Additionally, population pharmacokinetic evaluation of patients in controlled trials has revealed no evidence of clinically significant age or gender-related differences in the pharmacokinetics of ziprasidone. Dosage modifications for age or gender are, therefore, not recommended.
Close8.9 Smoking
Based on in vitro studies utilizing human liver enzymes, ziprasidone is not a substrate for CYP1A2; smoking should therefore not have an effect on the pharmacokinetics of ziprasidone. Consistent with these in vitro results, population pharmacokinetic evaluation has not revealed any significant pharmacokinetic differences between smokers and nonsmokers.
-
9 DRUG ABUSE AND DEPENDENCE9.3 Dependence - Ziprasidone has not been systematically studied, in animals or humans, for its potential for abuse, tolerance, or physical dependence. While the clinical trials did not reveal ...Close
9.3 Dependence
Ziprasidone has not been systematically studied, in animals or humans, for its potential for abuse, tolerance, or physical dependence. While the clinical trials did not reveal any tendency for drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which ziprasidone will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of ziprasidone misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
-
10 OVERDOSAGE10.1 Human Experience In premarketing trials involving more than 5400 patients and/or normal subjects, accidental or intentional overdosage of oral ziprasidone was documented in 10 ...
10.1 Human Experience
In premarketing trials involving more than 5400 patients and/or normal subjects, accidental or intentional overdosage of oral ziprasidone was documented in 10 patients. All of these patients survived without sequelae. In the patient taking the largest confirmed amount, 3,240 mg, the only symptoms reported were minimal sedation, slurring of speech, and transitory hypertension (200/95).
Adverse reactions reported with ziprasidone overdose included extrapyramidal symptoms, somnolence, tremor, and anxiety. [see Adverse Reactions (6.2)]
Close10.2 Management of Overdosage
In case of acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation. Intravenous access should be established, and gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizure, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of additive QT-prolonging effects that might be additive to those of ziprasidone.
Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids. If sympathomimetic agents are used for vascular support, epinephrine and dopamine should not be used, since beta stimulation combined with α1 antagonism associated with ziprasidone may worsen hypotension. Similarly, it is reasonable to expect that the alpha-adrenergic-blocking properties of bretylium might be additive to those of ziprasidone, resulting in problematic hypotension.
In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. There is no specific antidote to ziprasidone, and it is not dialyzable. The possibility of multiple drug involvement should be considered. Close medical supervision and monitoring should continue until the patient recovers.
-
11 DESCRIPTIONZiprasidone capsules, USP contains the active moiety, ziprasidone in the form of ziprasidone hydrochloride, USP salt. Ziprasidone is a psychotropic agent that is chemically unrelated to ...
Ziprasidone capsules, USP contains the active moiety, ziprasidone in the form of ziprasidone hydrochloride, USP salt. Ziprasidone is a psychotropic agent that is chemically unrelated to phenothiazine or butyrophenone antipsychotic agents. It has a molecular weight of 412.94 (free base), with the following chemical name: 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one. The molecular formula of C21H21ClN4OS (free base of ziprasidone) represents the following structural formula:
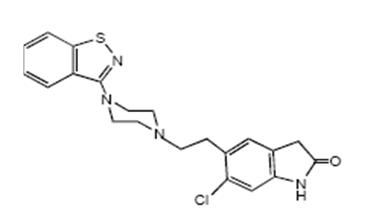
Ziprasidone capsules, USP contain a hydrochloride salt of ziprasidone. Chemically, ziprasidone hydrochloride USP is 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one, hydrochloride. The molecular formula is C21H21ClN4OS · HCl and its molecular weight is 449.40. Ziprasidone hydrochloride, USP is a light pink to pink colored powder.
Ziprasidone capsules, USP are supplied for oral administration in 20 mg, 40 mg, 60 mg, and 80 mg capsules. Ziprasidone capsules, USP contain ziprasidone hydrochloride USP, anhydrous lactose, magnesium stearate, polysorbate 80, povidone (PVK-30), pregelatinized starch and silicon dioxide. The components of the capsule shells are FD&C Blue #1, FD&C Red #3, gelatin, red iron oxide and titanium dioxide.
The capsule shells are imprinted with black ink. The components of black ink (Black SW-9008/SW-9009) are black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
Ziprasidone capsules meets USP Dissolution Test 3.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of ziprasidone in the treatment of the listed indications could be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 ...
12.1 Mechanism of Action
The mechanism of action of ziprasidone in the treatment of the listed indications could be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism.
12.2 Pharmacodynamics
Ziprasidone binds with relatively high affinity to the dopamine D2 and D3, serotonin 5HT2A, 5HT2C, 5HT1A, 5HT1D, and α1-adrenergic receptors (Ki s of 4.8, 7.2, 0.4, 1.3, 3.4, 2, and 10 nM, respectively), and with moderate affinity to the histamine H1 receptor (Ki=47 nM). Ziprasidone is an antagonist at the D2, 5HT2A, and 5HT1D receptors, and an agonist at the 5HT1A receptor. Ziprasidone inhibited synaptic reuptake of serotonin and norepinephrine. No appreciable affinity was exhibited for other receptor/binding sites tested, including the cholinergic muscarinic receptor (IC50 >1 mcM).
Close12.3 Pharmacokinetics
Oral Pharmacokinetics
Ziprasidone’s activity is primarily due to the parent drug. The multiple-dose pharmacokinetics of ziprasidone are dose-proportional within the proposed clinical dose range, and ziprasidone accumulation is predictable with multiple dosing. Elimination of ziprasidone is mainly via hepatic metabolism with a mean terminal half-life of about 7 hours within the proposed clinical dose range. Steady-state concentrations are achieved within one to three days of dosing. The mean apparent systemic clearance is 7.5 mL/min/kg. Ziprasidone is unlikely to interfere with the metabolism of drugs metabolized by cytochrome P450 enzymes.
Absorption: Ziprasidone is well absorbed after oral administration, reaching peak plasma concentrations in 6 to 8 hours. The absolute bioavailability of a 20 mg dose under fed conditions is approximately 60%. The absorption of ziprasidone is increased up to two-fold in the presence of food.
Distribution: Ziprasidone has a mean apparent volume of distribution of 1.5 L/kg. It is greater than 99% bound to plasma proteins, binding primarily to albumin and α1-acid glycoprotein. The in vitro plasma protein binding of ziprasidone was not altered by warfarin or propranolol, two highly protein-bound drugs, nor did ziprasidone alter the binding of these drugs in human plasma. Thus, the potential for drug interactions with ziprasidone due to displacement is minimal.
Metabolism and Elimination: Ziprasidone is extensively metabolized after oral administration with only a small amount excreted in the urine (<1%) or feces (<4%) as unchanged drug. Ziprasidone is primarily cleared via three metabolic routes to yield four major circulating metabolites, benzisothiazole (BITP) sulphoxide, BITP-sulphone, ziprasidone sulphoxide, and S-methyldihydroziprasidone. Approximately 20% of the dose is excreted in the urine, with approximately 66% being eliminated in the feces. Unchanged ziprasidone represents about 44% of total drug-related material in serum. In vitro studies using human liver subcellular fractions indicate that S-methyldihydroziprasidone is generated in two steps. These studies indicate that the reduction reaction is mediated primarily by chemical reduction by glutathione as well as by enzymatic reduction by aldehyde oxidase and the subsequent methylation is mediated by thiol methyltransferase. In vitro studies using human liver microsomes and recombinant enzymes indicate that CYP3A4 is the major CYP contributing to the oxidative metabolism of ziprasidone. CYP1A2 may contribute to a much lesser extent. Based on in vivo abundance of excretory metabolites, less than one-third of ziprasidone metabolic clearance is mediated by cytochrome P450 catalyzed oxidation and approximately two-thirds via reduction. There are no known clinically relevant inhibitors or inducers of aldehyde oxidase.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Lifetime carcinogenicity studies were conducted with ziprasidone in Long Evans rats and CD-1 mice. Ziprasidone was ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted with ziprasidone in Long Evans rats and CD-1 mice. Ziprasidone was administered for 24 months in the diet at doses of 2, 6, or 12 mg/kg/day to rats, and 50, 100, or 200 mg/kg/day to mice (0.1 to 0.6 and 1 to 5 times the maximum recommended human dose (MRHD) of 200 mg/day based on mg/m2 body surface area, respectively). In the rat study, there was no evidence of an increased incidence of tumors compared to controls. In male mice, there was no increase in incidence of tumors relative to controls. In female mice, there were dose-related increases in the incidences of pituitary gland adenoma and carcinoma, and mammary gland adenocarcinoma at all doses tested (50 to 200 mg/kg/day or 1 to 5 times the MRHD based on mg/m2 body surface area). Proliferative changes in the pituitary and mammary glands of rodents have been observed following chronic administration of other antipsychotic agents and are considered to be prolactin-mediated. Increases in serum prolactin were observed in a 1-month dietary study in female, but not male, mice at 100 and 200 mg/kg/day (or 2.5 and 5 times the MRHD based on mg/m2 body surface area). Ziprasidone had no effect on serum prolactin in rats in a 5-week dietary study at the doses that were used in the carcinogenicity study. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unknown [see Warnings and Precautions (5.14)].
Mutagenesis
Ziprasidone was tested in the Ames bacterial mutation assay, the in vitro mammalian cell gene mutation mouse lymphoma assay, the in vitro chromosomal aberration assay in human lymphocytes, and the in vivo chromosomal aberration assay in mouse bone marrow. There was a reproducible mutagenic response in the Ames assay in one strain of S. typhimurium in the absence of metabolic activation. Positive results were obtained in both the in vitro mammalian cell gene mutation assay and the in vitro chromosomal aberration assay in human lymphocytes.
Impairment of Fertility
Ziprasidone was shown to increase time to copulation in Sprague-Dawley rats in two fertility and early embryonic development studies at doses of 10 to 160 mg/kg/day (0.5 to 8 times the MRHD of 200 mg/day based on mg/m2 body surface area). Fertility rate was reduced at 160 mg/kg/day (8 times the MRHD based on mg/m2 body surface area). There was no effect on fertility at 40 mg/kg/day (2 times the MRHD based on mg/m2 body surface area). The effect on fertility appeared to be in the female since fertility was not impaired when males given 160 mg/kg/day (8 times the MRHD based on mg/m2 body surface area) were mated with untreated females.
-
14 CLINICAL STUDIES14.1 Schizophrenia The efficacy of oral ziprasidone in the treatment of schizophrenia was evaluated in 5 placebo-controlled studies, 4 short-term (4- and 6-week) trials and one ...
14.1 Schizophrenia
The efficacy of oral ziprasidone in the treatment of schizophrenia was evaluated in 5 placebo-controlled studies, 4 short-term (4- and 6-week) trials and one maintenance trial. All trials were in adult inpatients, most of whom met DSM III-R criteria for schizophrenia. Each study included 2 to 3 fixed doses of ziprasidone as well as placebo. Four of the 5 trials were able to distinguish ziprasidone from placebo; one short-term study did not. Although a single fixed-dose haloperidol arm was included as a comparative treatment in one of the three short-term trials, this single study was inadequate to provide a reliable and valid comparison of ziprasidone and haloperidol.
Several instruments were used for assessing psychiatric signs and symptoms in these studies. The Brief Psychiatric Rating Scale (BPRS) and the Positive and Negative Syndrome Scale (PANSS) are both multi-item inventories of general psychopathology usually used to evaluate the effects of drug treatment in schizophrenia. The BPRS psychosis cluster (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) is considered a particularly useful subset for assessing actively psychotic schizophrenic patients. A second widely used assessment, the Clinical Global Impression (CGI), reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient. In addition, the Scale for Assessing Negative Symptoms (SANS) was employed for assessing negative symptoms in one trial.
The results of the oral ziprasidone trials in schizophrenia follow:
- In a 4-week, placebo-controlled trial (n=139) comparing 2 fixed doses of ziprasidone (20 and 60 mg twice daily) with placebo, only the 60 mg dose was superior to placebo on the BPRS total score and the CGI severity score. This higher dose group was not superior to placebo on the BPRS psychosis cluster or on the SANS.
- In a 6-week, placebo-controlled trial (n=302) comparing 2 fixed doses of ziprasidone (40 and 80 mg twice daily) with placebo, both dose groups were superior to placebo on the BPRS total score, the BPRS psychosis cluster, the CGI severity score and the PANSS total and negative subscale scores. Although 80 mg twice daily had a numerically greater effect than 40 mg twice daily, the difference was not statistically significant.
- In a 6-week, placebo-controlled trial (n=419) comparing 3 fixed doses of ziprasidone (20, 60, and 100 mg twice daily) with placebo, all three dose groups were superior to placebo on the PANSS total score, the BPRS total score, the BPRS psychosis cluster, and the CGI severity score. Only the 100 mg twice daily dose group was superior to placebo on the PANSS negative subscale score. There was no clear evidence for a dose-response relationship within the 20 mg twice daily to 100 mg twice daily dose range.
- In a 4-week, placebo-controlled trial (n=200) comparing 3 fixed doses of ziprasidone (5, 20, and 40 mg twice daily), none of the dose groups was statistically superior to placebo on any outcome of interest.
- A study was conducted in stable chronic or subchronic (CGI-S <5 at baseline) schizophrenic inpatients (n=294) who had been hospitalized for not less than two months. After a 3-day single-blind placebo run-in, subjects were randomized to one of 3 fixed doses of ziprasidone (20 mg, 40 mg, or 80 mg twice daily) or placebo and observed for relapse. Patients were observed for “impending psychotic relapse,” defined as CGI-improvement score of >6 (much worse or very much worse) and/or scores >6 (moderately severe) on the hostility or uncooperativeness items of the PANSS on two consecutive days. Ziprasidone was significantly superior to placebo in time to relapse, with no significant difference between the different dose groups. There were insufficient data to examine population subsets based on age and race. Examination of population subsets based on gender did not reveal any differential responsiveness.
Close14.2 Bipolar I Disorder (Acute Mixed or Manic Episodes and Maintenance Treatment as an Adjunct to Lithium or Valproate)
Acute Manic and Mixed Episodes Associated With Bipolar I Disorder
The efficacy of ziprasidone was established in 2 placebo-controlled, double-blind, 3-week monotherapy studies in patients meeting DSM-IV criteria for bipolar I disorder, manic or mixed episode with or without psychotic features. Primary rating instruments used for assessing manic symptoms in these trials were: (1) the Mania Rating Scale (MRS), which is derived from the Schedule for Affective Disorders and Schizophrenia-Change Version (SADS-CB) with items grouped as the Manic Syndrome subscale (elevated mood, less need for sleep, excessive energy, excessive activity, grandiosity), the Behavior and Ideation subscale (irritability, motor hyperactivity, accelerated speech, racing thoughts, poor judgment) and impaired insight; and (2) the Clinical Global Impression-Severity of Illness Scale (CGI-S), which was used to assess the clinical significance of treatment response.
The results of the oral ziprasidone trials in adult bipolar I disorder, manic/mixed episode follow: in a 3-week placebo-controlled trial (n=210), the dose of ziprasidone was 40 mg twice daily on Day 1 and 80 mg twice daily on Day 2. Titration within the range of 40-80 mg twice daily (in 20 mg twice daily increments) was permitted for the duration of the study. Ziprasidone was significantly more effective than placebo in reduction of the MRS total score and the CGI-S score. The mean daily dose of ziprasidone in this study was 132 mg. In a second 3-week placebo-controlled trial (n=205), the dose of ziprasidone was 40 mg twice daily on Day 1. Titration within the range of 40-80 mg twice daily (in 20 mg twice daily increments) was permitted for the duration of study (beginning on Day 2). Ziprasidone was significantly more effective than placebo in reduction of the MRS total score and the CGI-S score. The mean daily dose of ziprasidone in this study was 112 mg.
Maintenance Therapy
The efficacy of ziprasidone as adjunctive therapy to lithium or valproate in the maintenance treatment of bipolar I disorder was established in a placebo-controlled trial in patients who met DSM-IV criteria for bipolar I disorder. The trial included patients whose most recent episode was manic or mixed, with or without psychotic features. In the open-label phase, patients were required to be stabilized on ziprasidone plus lithium or valproic acid for at least 8 weeks in order to be randomized. In the double-blind randomized phase, patients continued treatment with lithium or valproic acid and were randomized to receive either ziprasidone (administered twice daily totaling 80 mg to 160 mg per day) or placebo. Generally, in the maintenance phase, patients continued on the same dose on which they were stabilized during the stabilization phase. The primary endpoint in this study was time to recurrence of a mood episode (manic, mixed or depressed episode) requiring intervention, which was defined as any of the following: discontinuation due to a mood episode, clinical intervention for a mood episode (e.g., initiation of medication or hospitalization), or Mania Rating Scale score ≥18 or a MADRS score ≥ 8 (on 2 consecutive assessments no more than 10 days apart). A total of 584 subjects were treated in the open-label stabilization period. In the double-blind randomization period, 127 subjects were treated with ziprasidone, and 112 subjects were treated with placebo. Ziprasidone was superior to placebo in increasing the time to recurrence of a mood episode. The types of relapse events observed included depressive, manic, and mixed episodes. Depressive, manic, and mixed episodes accounted for 53%, 34%, and 13%, respectively, of the total number of relapse events in the study.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZiprasidone capsules, USP 20 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and Flesh opaque body, imprinted “RDY” on ...
Ziprasidone capsules, USP 20 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and Flesh opaque body, imprinted “RDY” on cap and “256” on body with black ink, and are supplied in bottles of 60’s.
Bottles of 60 NDC 16714-835-01
Ziprasidone capsules, USP 40 mg (ziprasidone), are light pink to brown granular powder filled in size “4” hard gelatin capsules having Lavender opaque cap and LT Turquoise blue opaque body, imprinted “RDY” on cap and “257” on body with black ink, and are supplied in bottles of 60’s.
Bottles of 60 NDC 16714-836-01
Ziprasidone capsules, USP 60 mg (ziprasidone), are light pink to brown granular powder filled in size “3” hard gelatin capsules having Flesh opaque cap and Flesh opaque body, imprinted “RDY” on cap and “258” on body with black ink, and are supplied in bottles of 60’s.
Bottles of 60 NDC 16714-837-01
Ziprasidone capsules, USP 80 mg (ziprasidone), are light pink to brown granular powder filled in size “2” hard gelatin capsules having LT Turquoise blue opaque cap and Flesh opaque body, imprinted “RDY” on cap and “259” on body with black ink, and are supplied in bottles of 60’s.
Bottles of 60 NDC 16714-838-01
Ziprasidone capsules, USP should be stored at 20° to 25°C (68° to 77°F);excursions permitted to 15° to 30°C(59° to 86°F) [See USP Controlled Room Temperature].
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Administration Information for Capsules - Advise patients to take ziprasidone capsules whole. Do not open ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Information for Capsules
Advise patients to take ziprasidone capsules whole. Do not open, crush, or chew the capsules. Instruct patients to take ziprasidone capsules with food for optimal absorption. The absorption of ziprasidone is increased up to two-fold in the presence of food[seeDosage and Administration (2.1), Drug Interactions (7.10),andClinical Pharmacology (12.3)].
Instruct patients to take ziprasidone capsules with food for optimal absorption. The absorption of ziprasidone is increased up to two-fold in the presence of food [see Drug Interactions (7.10) and Clinical Pharmacology (12.3)].
QTc Prolongation
Advise patients to inform their health care providers of the following: History of QT prolongation; recent acute myocardial infarction; uncompensated heart failure; prescription of other drugs that have demonstrated QT prolongation; risk for significant electrolyte abnormalities; and history of cardiac arrhythmia [see Contraindications (4.1) and Warnings and Precautions (5.3)].
Instruct patients to report the onset of any conditions that put them at risk for significant electrolyte disturbances, hypokalemia in particular, including but not limited to the initiation of diuretic therapy or prolonged diarrhea. In addition, instruct patients to report symptoms such as dizziness, palpitations, or syncope to the prescriber [see Warnings and Precautions (5.3)].
Severe Cutaneous Adverse Reactions Instruct patients to report to their health care provider at the earliest onset any signs or symptoms that may be associated with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) or with severe cutaneous adverse reactions, such as Stevens-Johnson syndrome [see Warnings and Precautions (5.5)].
Pregnancy
Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with ziprasidone capsules. Advise patients that ziprasidone capsules may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ziprasidone capsules during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using ziprasidone capsules to monitor infants for excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors, and abnormal muscle movements) and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that ziprasidone capsules may impair fertility due to an increase in serum prolactin levels. The effects on fertility are reversible [see Warnings and Precautions (5.15) and Use in Specific Populations (8.3)].
Brands listed are the trademarks of their respective owners.
Close -
PATIENT SUMMARY OF INFORMATIONABOUT
Ziprasidone Capsules, USP - (zi pras' i done) Information for patients taking ziprasidone capsules or their caregivers - This summary contains important information about ziprasidone capsules ...
Ziprasidone Capsules, USP
(zi pras' i done)
Information for patients taking ziprasidone capsules or their caregivers
This summary contains important information about ziprasidone capsules. It is not meant to take the place of your doctor’s instructions. Read this information carefully before you take ziprasidone capsules. Ask your doctor or pharmacist if you do not understand any of this information or if you want to know more about ziprasidone capsules.
What are ziprasidone capsules?
Ziprasidone capsules are a type of prescription medicine called a psychotropic, also known as an atypical antipsychotic. Ziprasidone capsules can be used to treat symptoms of schizophrenia and acute manic or mixed episodes associated with bipolar disorder. Ziprasidone capsules can also be used as maintenance treatment of bipolar disorder when added to lithium or valproate.
Who should take ziprasidone capsules?
Only your doctor can know if ziprasidone capsules are right for you. Ziprasidone capsules may be prescribed for you if you are an adult with schizophrenia or bipolar disorder. Symptoms of schizophrenia may include:
- hearing voices, seeing things, or sensing things that are not there (hallucinations)
- beliefs that are not true (delusions)
- unusual suspiciousness (paranoia)
- becoming withdrawn from family and friends
Symptoms of manic or mixed episodes of bipolar disorder may include:
• extremely high or irritable mood
• increased energy, activity, and restlessness
• racing thoughts or talking very fast
• easily distracted
• little need for sleep
If you show a response to ziprasidone capsules, your symptoms may improve. If you continue to take ziprasidone capsules there is less chance of your symptoms returning. Do not stop taking the capsules even when you feel better without first discussing it with your doctor.
It is also important to remember that ziprasidone capsules should be taken with food.
What is the most important safety information I should know about ziprasidone capsules?
Ziprasidone capsules are not approved for the treatment of patients with dementia-related psychosis. Elderly patients with a diagnosis of psychosis related to dementia treated with antipsychotics are at an increased risk of death when compared to patients who are treated with placebo (a sugar pill).
Ziprasidone capsules are an effective drug to treat the symptoms of schizophrenia and the manic or mixed episodes of bipolar disorder. However, one potential side effect is that it may change the way the electrical current in your heart works more than some other drugs. The change is small and it is not known whether this will be harmful, but some other drugs that cause this kind of change have in rare cases caused dangerous heart rhythm abnormalities. Because of this, ziprasidone capsules should be used only after your doctor has considered this risk for ziprasidone capsules against the risks and benefits of other medications available for treating schizophrenia or bipolar manic and mixed episodes.
Your risk of dangerous changes in heart rhythm can be increased if you are taking certain other medicines and if you already have certain abnormal heart conditions. Therefore, it is important to tell your doctor about any other medicines that you take, including non-prescription medicines, supplements, and herbal medicines. You must also tell your doctor about any heart problems you have or have had.
Who should NOT take ziprasidone capsules?
Elderly patients with a diagnosis of psychosis related to dementia. Ziprasidone capsules are not approved for the treatment of these patients.
Anything that can increase the chance of a heart rhythm abnormality should be avoided. Therefore, do not take ziprasidone capsules if: You have certain heart diseases, for example, long QT syndrome, a recent heart attack, severe heart failure, or certain irregularities of heart rhythm (discuss the specifics with your doctor) You are currently taking medications that should not be taken in combination with ziprasidone, for example, dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, pimozide, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol or tacrolimus.
What to tell your doctor before you start ziprasidone capsules?
Only your doctor can decide if ziprasidone capsules are right for you. Before you start ziprasidone capsules, be sure to tell your doctor if you:
-
have had any problem with the way your heart beats or any heart related illness or disease
-
any family history of heart disease, including recent heart attack
-
have had any problem with fainting or dizziness
-
are taking or have recently taken any prescription medicines
-
are taking any over-the-counter medicines you can buy without a prescription, including natural/herbal remedies
-
have had any problems with your liver
-
are pregnant, might be pregnant, or plan to get pregnant
-
are breastfeeding or plan to breastfeed
-
are allergic to any medicines
-
have ever had an allergic reaction to ziprasidone or any of the other ingredients of ziprasidone capsules. Ask your doctor or pharmacist for a list of these ingredients
-
have low levels of potassium or magnesium in your blood
Your doctor may want you to get additional laboratory tests to see if ziprasidone capsules are an appropriate treatment for you.
Ziprasidone capsules and other medicines
There are some medications that may be unsafe to use when taking ziprasidone capsules, and there are some medicines that can affect how well ziprasidone capsules works. While you are on ziprasidone capsules, check with your doctor before starting any new prescription or over-the-counter medications, including natural/herbal remedies.
How to take ziprasidone capsules
• Take ziprasidone capsules only as directed by your doctor.
• Swallow ziprasidone capsules whole. Do not chew, crush, or open the capsules.
• Take ziprasidone capsules with food.
• It is best to take ziprasidone capsules at the same time each day.
• Ziprasidone capsules may take a few weeks to work. It is important to be patient.
• Do not change your dose or stop taking your medicine without your doctor’s approval.
• Remember to keep taking your capsules, even when you feel better.
Possible side effects
Because these problems could mean you’re having a heart rhythm abnormality, contact your doctor IMMEDIATELY if you:
-
Faint or lose consciousness
-
Feel a change in the way that your heart beats (palpitations)
Common side effects of ziprasidone capsules include the following and should also be discussed with your doctor if they occur:
Adults:
-
Feeling unusually tired or sleepy
-
Nausea or upset stomach
-
Constipation
-
Dizziness
-
Restlessness
-
Abnormal muscle movements, including tremor, shuffling, and uncontrolled involuntary movements
-
Diarrhea
-
Rash
-
Increased cough / runny nose
If you develop any side effects that concern you, talk with your doctor. It is particularly important to tell your doctor if you have diarrhea, vomiting, or another illness that can cause you to lose fluids. Your doctor may want to check your blood to make sure that you have the right amount of important salts after such illnesses.
For a list of all side effects that have been reported, ask your doctor or pharmacist for the ziprasidone capsules Professional Package Insert.
What to do for an overdose
In case of an overdose, call your doctor or poison control center right away or go to the nearest emergency room.
Other Important Safety Information
A serious condition called neuroleptic malignant syndrome (NMS) can occur with all antipsychotic medications including ziprasidone capsules. Signs of NMS include very high fever, rigid muscles, shaking, confusion, sweating, or increased heart rate and blood pressure. NMS is a rare but serious side effect that could be fatal. Therefore, tell your doctor if you experience any of these signs.
Delayed-onset drug reaction called drug reaction with eosinophilia and systemic symptoms (DRESS) can occur with ziprasidone. Signs of DRESS may include rash, fever, and swollen lymph nodes. Other severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome can occur with ziprasidone. Signs of Stevens-Johnson syndrome may include rash with blisters which could include ulcers in mouth, skin shedding, fever and target-like spots in the skin. DRESS and other SCAR are sometimes fatal; therefore, tell your doctor immediately if you experience any of these signs.
Adverse reactions related to high blood sugar (hyperglycemia), sometimes serious, have been reported in patients treated with atypical antipsychotics. There have been few reports of hyperglycemia or diabetes in patients treated with ziprasidone capsules, and it is not known if ziprasidone capsules are associated with these reactions. Patients treated with an atypical antipsychotic should be monitored for symptoms of hyperglycemia.
Dizziness caused by a drop in your blood pressure may occur with ziprasidone capsules, especially when you first start taking this medication or when the dose is increased. If this happens, be careful not to stand up too quickly, and talk to your doctor about the problem.
Before taking ziprasidone capsules, tell your doctor if you
-
are pregnant or plan on becoming pregnant.
-
If you become pregnant while receiving ziprasidone capsules, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-andresearch-programs/pregnancyregistry
-
are breastfeeding or plan to breastfeed. Ziprasidone capsules can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive ziprasidone capsules.
Because ziprasidone capsules can cause sleepiness, be careful when operating machinery or driving a motor vehicle.
Since medications of the same drug class as ziprasidone capsules may interfere with the ability of the body to adjust to heat, it is best to avoid situations involving high temperature or humidity.
It is best to avoid consuming alcoholic beverages while taking ziprasidone capsules. Call your doctor immediately if you take more than the amount of ziprasidone capsules prescribed by your doctor. Ziprasidone capsules have not been shown to be safe or effective in the treatment of children and teenagers under the age of 18 years old.
Keep ziprasidone capsules and all medicines out of the reach of children.
How to store ziprasidone capsules
Store ziprasidone capsules, USP at room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
For more information about ziprasidone capsules
This sheet is only a summary. Ziprasidone capsules are a prescription medicine and only your doctor can decide if it is right for you. If you have any questions or want more information about ziprasidone capsules, talk with your doctor or pharmacist or call, 1-800-206-7821.
Manufactured for:
NorthStar Rx LLC Memphis, TN 38141.
Manufactured by:
Dr. Reddy's Laboratories Limited
Bachupally - 500 090 INDIA.
Revised:06/2023
Close -
Package Label. Principal Display PanelZiprasidone Capsules, USP 20 mg - Container Label
-
PRINCIPAL DISPLAY PANELZiprasidone Capsules, USP 40 mg - Container Label
-
PRINCIPAL DISPLAY PANELZiprasidone Capsules, USP 60 mg - Container Label
-
PRINCIPAL DISPLAY PANELZiprasidone Capsules, USP 80 mg - Container Label
-
INGREDIENTS AND APPEARANCEProduct Information
ZIPRASIDONE ziprasidone capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16714-835 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ziprasidone hydrochloride (UNII: 216X081ORU) (ziprasidone - UNII:6UKA5VEJ6X) ziprasidone 20 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) magnesium stearate (UNII: 70097M6I30) polysorbate 80 (UNII: 6OZP39ZG8H) povidone k30 (UNII: U725QWY32X) starch, corn (UNII: O8232NY3SJ) silicon dioxide (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) gelatin (UNII: 2G86QN327L) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) ferrosoferric oxide (UNII: XM0M87F357) butyl alcohol (UNII: 8PJ61P6TS3) alcohol (UNII: 3K9958V90M) isopropyl alcohol (UNII: ND2M416302) potassium hydroxide (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) ammonia (UNII: 5138Q19F1X) Product Characteristics Color PURPLE (Lavender opaque cap and flesh opaque body) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RDY;256 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-835-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077565 07/26/2018 ZIPRASIDONE ziprasidone capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16714-836 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ziprasidone hydrochloride (UNII: 216X081ORU) (ziprasidone - UNII:6UKA5VEJ6X) ziprasidone 40 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) magnesium stearate (UNII: 70097M6I30) polysorbate 80 (UNII: 6OZP39ZG8H) povidone k30 (UNII: U725QWY32X) starch, corn (UNII: O8232NY3SJ) silicon dioxide (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) gelatin (UNII: 2G86QN327L) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) ferrosoferric oxide (UNII: XM0M87F357) butyl alcohol (UNII: 8PJ61P6TS3) alcohol (UNII: 3K9958V90M) isopropyl alcohol (UNII: ND2M416302) potassium hydroxide (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) ammonia (UNII: 5138Q19F1X) Product Characteristics Color PURPLE (Lavender opaque cap and LT turquoise blue opaque body) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RDY;257 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-836-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077565 07/26/2018 ZIPRASIDONE ziprasidone capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16714-837 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ziprasidone hydrochloride (UNII: 216X081ORU) (ziprasidone - UNII:6UKA5VEJ6X) ziprasidone 60 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) magnesium stearate (UNII: 70097M6I30) polysorbate 80 (UNII: 6OZP39ZG8H) povidone k30 (UNII: U725QWY32X) starch, corn (UNII: O8232NY3SJ) silicon dioxide (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) gelatin (UNII: 2G86QN327L) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) ferrosoferric oxide (UNII: XM0M87F357) butyl alcohol (UNII: 8PJ61P6TS3) alcohol (UNII: 3K9958V90M) isopropyl alcohol (UNII: ND2M416302) potassium hydroxide (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) ammonia (UNII: 5138Q19F1X) Product Characteristics Color BROWN (Flesh opaque cap and Flesh opaque body) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code RDY;258 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-837-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077565 07/26/2018 ZIPRASIDONE ziprasidone capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16714-838 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ziprasidone hydrochloride (UNII: 216X081ORU) (ziprasidone - UNII:6UKA5VEJ6X) ziprasidone 80 mg Inactive Ingredients Ingredient Name Strength anhydrous lactose (UNII: 3SY5LH9PMK) magnesium stearate (UNII: 70097M6I30) polysorbate 80 (UNII: 6OZP39ZG8H) povidone k30 (UNII: U725QWY32X) starch, corn (UNII: O8232NY3SJ) silicon dioxide (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) gelatin (UNII: 2G86QN327L) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) ferrosoferric oxide (UNII: XM0M87F357) butyl alcohol (UNII: 8PJ61P6TS3) alcohol (UNII: 3K9958V90M) isopropyl alcohol (UNII: ND2M416302) potassium hydroxide (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) ammonia (UNII: 5138Q19F1X) Product Characteristics Color BLUE (LT Turquoise blue opaque cap and Flesh opaque body) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code RDY;259 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16714-838-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077565 07/26/2018 Labeler - NorthStar Rx LLC (830546433)
CloseEstablishment Name Address ID/FEI Business Operations Dr. Reddy's Laboratories Limited - FTO 3 918608162 analysis(16714-835, 16714-836, 16714-837, 16714-838) , manufacture(16714-835, 16714-836, 16714-837, 16714-838)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ZIPRASIDONE capsule
Number of versions: 8
RxNorm
ZIPRASIDONE capsule
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 313776 | ziprasidone HCl 40 MG Oral Capsule | PSN |
| 2 | 313776 | ziprasidone 40 MG Oral Capsule | SCD |
| 3 | 313776 | ziprasidone (as ziprasidone hydrochloride monohydrate) 40 MG Oral Capsule | SY |
| 4 | 313777 | ziprasidone HCl 60 MG Oral Capsule | PSN |
| 5 | 313777 | ziprasidone 60 MG Oral Capsule | SCD |
| 6 | 313777 | ziprasidone (as ziprasidone HCl monohydrate) 60 MG Oral Capsule | SY |
| 7 | 313778 | ziprasidone HCl 80 MG Oral Capsule | PSN |
| 8 | 313778 | ziprasidone 80 MG Oral Capsule | SCD |
| 9 | 313778 | ziprasidone (as ziprasidone hydrochloride monohydrate) 80 MG Oral Capsule | SY |
| 10 | 314286 | ziprasidone HCl 20 MG Oral Capsule | PSN |
| 11 | 314286 | ziprasidone 20 MG Oral Capsule | SCD |
| 12 | 314286 | ziprasidone (as ziprasidone HCl monohydrate) 20 MG Oral Capsule | SY |
Get Label RSS Feed for this Drug
ZIPRASIDONE capsule
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=0071a228-21cc-56a6-dcc9-a7a6ceea758c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ZIPRASIDONE capsule
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 16714-835-01 |
| 2 | 16714-836-01 |
| 3 | 16714-837-01 |
| 4 | 16714-838-01 |