Label: TRANEXAMIC ACID injection, solution
- NDC Code(s): 83634-401-10, 83634-401-41
- Packager: Avenacy, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRANEXAMIC ACID INJECTION safely and effectively. See full prescribing information for TRANEXAMIC ACID INJECTION. TRANEXAMIC ACID ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Tranexamic acid injection, USP is indicated in patients with hemophilia for short-term use (2 to 8 days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - The recommended dose of tranexamic acid injection is 10 mg/kg actual body weight intravenously administered as a single-dose, immediately before tooth extractions ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 1,000 mg tranexamic acid (100 mg per mL) clear and colorless solution in 10 mL single-dose vials.
-
4 CONTRAINDICATIONS
Tranexamic acid injection, USP is contraindicated: In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Risk - Tranexamic acid injection is contraindicated in patients with active intravascular clotting. Tranexamic acid is an antifibrinolytic and may increase the risk of ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Thromboembolic Risk [see Warnings and Precautions (5.1)] Seizures [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS
7.1 Prothrombotic Medical Products - Avoid concomitant use of tranexamic acid injection with medical products that are prothrombotic because concomitant use can further increase the risk of ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Available data from published studies, case series and case reports with tranexamic acid use in pregnant women in the second and third trimester and at the time ...
-
10 OVERDOSAGE
Cases of overdosage of tranexamic acid injection have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., nausea, vomiting, diarrhea; hypotensive, e.g. ...
-
11 DESCRIPTION
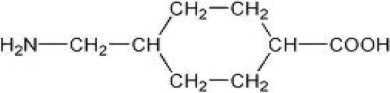
Tranexamic acid is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid, USP is a white crystalline powder. The structural formula is - Empirical ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Tranexamic acid was not carcinogenic in a 2-year study in rats and mice at oral doses up to 3 and 5.3 g/kg/day, which are ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tranexamic Acid Injection, USP is supplied as follows: Tranexamic Acid Injection, USP - NDC(100 mg per mL)Package Factor - 83634-401-10 - 1,000 mg per 10 mL Single-Dose Vial - 10 vials per ...
-
17 PATIENT COUNSELING INFORMATION
Thromboembolic Risk - Inform patients that tranexamic acid injection may increase the risk of venous and arterial thrombosis or thromboembolism and to contact their healthcare provider for any ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY PANEL – Vial Label - NDC 83634-401-41 - Tranexamic Acid Injection, USP - 1,000 mg per 10 mL (100 mg per mL) Intravenous Use Only - 10 mL Single-Dose Vial - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information