Label: KIZO LAB UV DEFENSE CLEAR SUNSCREN SPF50- avobenzone,homosalate,octisalate,octocrylene gel
- NDC Code(s): 81208-203-02
- Packager: YB CHEMIA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- USES
- WARNINGS
- Keep Out of Reach
-
DIRECTIONS
Apply liberally 15 minutes before sun exposure
Rub in well.
Reapply at least every 2 hours
Children under 6 months: Ask a doctor
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF of 15 or higher and other sun protection measures including:Limit time in the sun, especially 10 a.m. to 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses. - OTHER INFORMATION
-
Inactive Ingredients
Acetyl Hexapeptide-1, C12-15 Alkyl Benzoate, C18-36 Acid Glycol Ester, C18-36 Acid Triglyceride, Capryloyl Glycerin/Sebacic Acid Copolymer, Capryloyl Glycine, Caprylyl Glycol, Coprinus Comatus (Mushroom) Extract, Cordyceps Sinensis Extract, Dicaprylyl Carbonate, Dimethicone Crosspolymer-3, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Ethylhexyl Methoxycrylene, Ethylhexylglycerin, Ganoderma Lucidum (Mushroom) Extract, Glycerin, Grifola Frondosa Extract, Isododecane, Isohexadecane, Isopropyl Palmitate, Lecithin, Meadowfoam Estolide, Neopentyl Glycol Diheptanoate, Octyldodecyl Neopentanoate, Palmitoyl Tetrapeptide-7, Palmitoyl Tripeptide-1, Polyglutamic Acid, Polymethylsilsesquioxane, Rhododendron Ferrugineum Extract, Sorbitan Laurate, Terminalia Chebula Fruit Extract, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Undecylenoyl Glycine, Water.
- DESCRIPTION
- Directions:
- Indications & Usage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KIZO LAB UV DEFENSE CLEAR SUNSCREN SPF50
avobenzone,homosalate,octisalate,octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81208-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) 1 g in 100 g CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) 1 g in 100 g CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1 g in 100 g DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) 1 g in 100 g DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) 1 g in 100 g DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) 1 g in 100 g ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) 1 g in 100 g ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) 1 g in 100 g ISOHEXADECANE (UNII: 918X1OUF1E) 1 g in 100 g WATER (UNII: 059QF0KO0R) 1 g in 100 g DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) 1 g in 100 g PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) 1 g in 100 g POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) 1 g in 100 g ISODODECANE (UNII: A8289P68Y2) 1 g in 100 g ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) 1 g in 100 g LECITHIN, SOYBEAN (UNII: 1DI56QDM62) 1 g in 100 g MEADOWFOAM ESTOLIDE (UNII: 3HH93SL2H9) 1 g in 100 g PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) 1 g in 100 g UNDECYLENOYL GLYCINE (UNII: 4D20464K2J) 1 g in 100 g ACETYL HEXAPEPTIDE-1 (UNII: 49ZWR266MZ) 1 g in 100 g C18-36 ACID TRIGLYCERIDE (UNII: ZRA72DR3R7) 1 g in 100 g ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 1 g in 100 g NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) 1 g in 100 g OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) 1 g in 100 g TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) 1 g in 100 g Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81208-203-02 42.5 g in 1 TUBE; Type 0: Not a Combination Product 07/12/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/12/2023 Labeler - YB CHEMIA LLC (095778265)

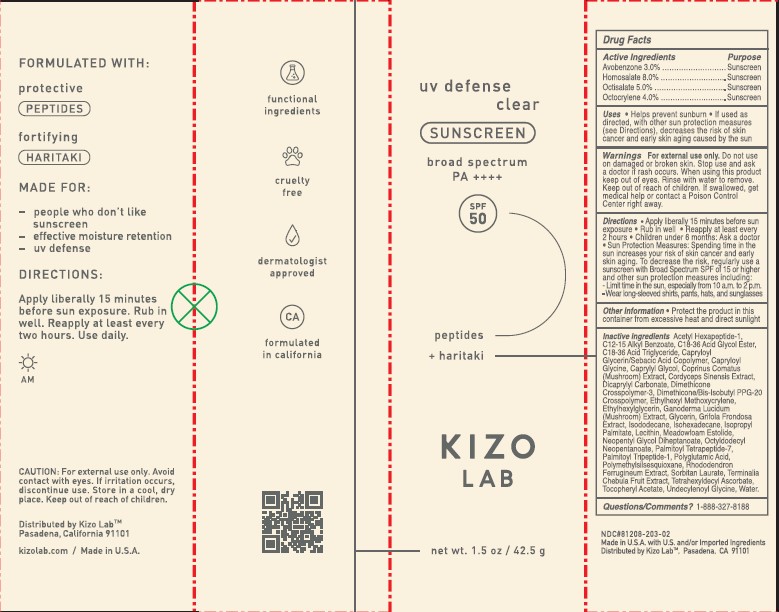
 KIZO LAB UV DEFENSE SPF50
KIZO LAB UV DEFENSE SPF50
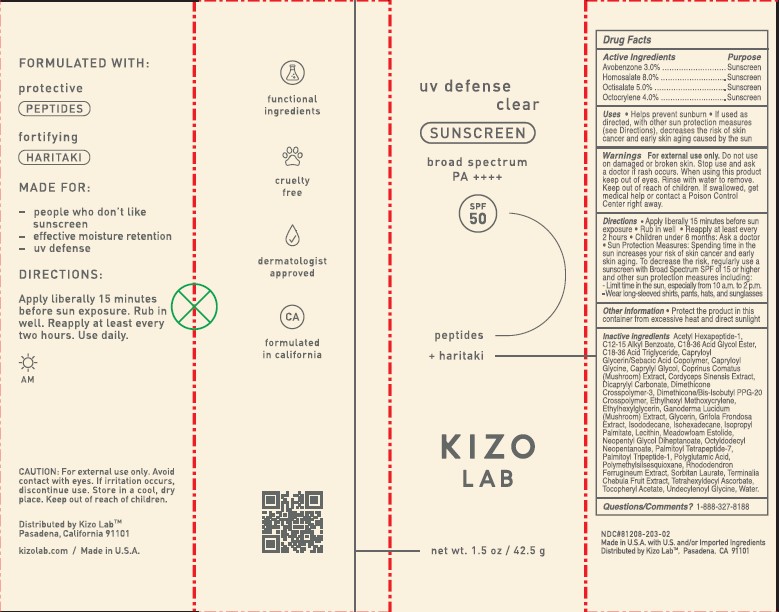 KIZO SPF 50 BOX
KIZO SPF 50 BOX