Label: METOCLOPRAMIDE solution
- NDC Code(s): 60687-837-42, 60687-837-48, 60687-837-56
- Packager: American Health Packaging
- This is a repackaged label.
- Source NDC Code(s): 62559-190
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: TARDIVE DYSKINESIA
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia.
See WARNINGS
Close -
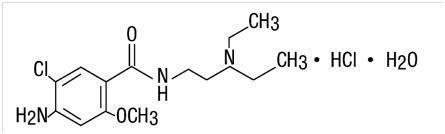
DESCRIPTIONMetoclopramide Oral Solution USP is an orange-colored, vanilla-flavored, palatable, aromatic, sugar-free liquid for oral administration. Each 5 mL (teaspoonful) contains: Metoclopramide base (as ...
-
CLINICAL PHARMACOLOGYMetoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary or pancreatic secretions. Its mode of action is unclear. It seems to sensitize tissues ...
-
INDICATIONS AND USAGEThe use of Metoclopramide Oral Solution is recommended for adults only. Therapy should not exceed 12 weeks in duration. Symptomatic Gastroesophageal Reflux - Metoclopramide Oral Solution is ...
-
CONTRAINDICATIONSMetoclopramide should not be used whenever stimulation of gastrointestinal motility might be dangerous, e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or ...
-
WARNINGSMental depression has occurred in patients with and without prior history of depression. Symptoms have ranged from mild to severe and have included suicidal ideation and suicide. Metoclopramide ...
-
PRECAUTIONSGeneral - In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, caution should be exercised when metoclopramide is used in ...
-
ADVERSE REACTIONSIn general, the incidence of adverse reactions correlates with the dose and duration of metoclopramide administration. The following reactions have been reported, although in most instances, data ...
-
OVERDOSAGESymptoms of overdosage may include drowsiness, disorientation and extrapyramidal reactions. Anticholinergic or antiparkinson drugs or antihistamines with anticholinergic properties may be helpful ...
-
DOSAGE AND ADMINISTRATIONTherapy with metoclopramide oral solution should not exceed 12 weeks in duration. For the Relief of Symptomatic Gastroesophageal Reflux - Administer from 10 mg to 15 mg metoclopramide orally ...
-
HOW SUPPLIEDMetoclopramide Oral Solution USP, 5 mg metoclopramide base (as the monohydrochloride monohydrate) per 5 mL (teaspoonful) is available as an orange-colored, vanilla-flavored, palatable, aromatic ...
-
PACKAGING INFORMATIONAmerican Health Packaging unit dose cups (see - How Supplied section) contain drug product from ANI Pharmaceuticals, Inc. as follows: (10 mg / 10 mL / 100 UD) NDC 60687‐837‐56 packaged from ...
-
Medication Guide8483756/0324F - Metoclopramide (met-o-KLO-pra-mide) Oral Solution - Read the Medication Guide that comes with Metoclopramide before you start taking it and each time you get a refill. There may be ...
-
Package/Label Display Panel – LabelMetoclopramide - Oral Solution USP - Rx Only - FOR INSTITUTIONAL USE ONLY - PHARMACIST: Dispense with - Medication Guide to each patient. Store at 20° to 25°C (68° to 77°F) ...
-
Package/Label Display Panel – Cup Lid – 10 mg/10 mLRx Only - NDC 60687- 837-42 - Metoclopramide - Oral Solution USP - 10 mg/10 mL - Sugar-free and Alcohol-free - Delivers 10 mL - PROTECT FROM FREEZING. See package insert for full ...
-
INGREDIENTS AND APPEARANCEProduct Information