Label: COMPLETENATE CHEWABLE- .beta.-carotene, ascorbic acid, sodium ascorbate, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, and ferrous fumarate tablet
- NHRIC Code(s): 13811-014-90
- Packager: Trigen Laboratories, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SUPPLEMENT FACTS
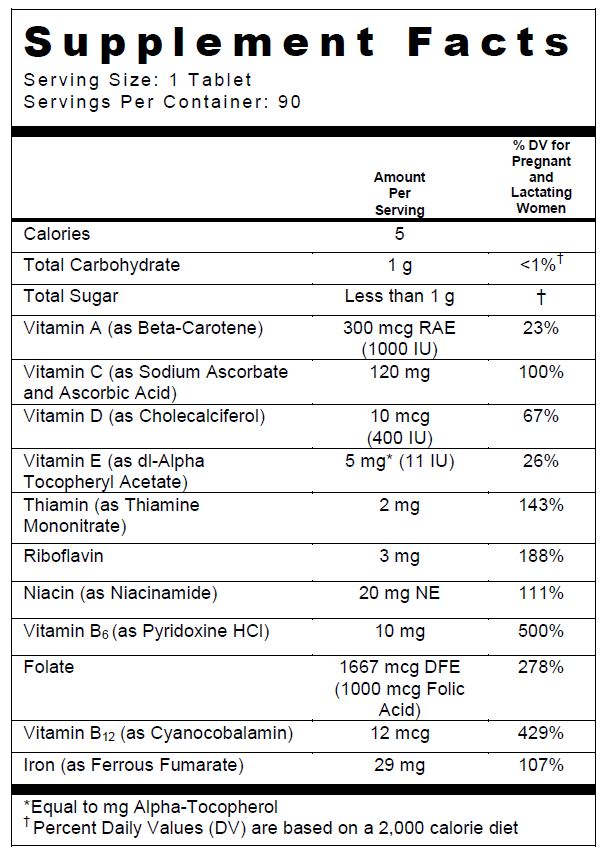
Other Ingredients: Sorbitol, Sucrose, Natural Orange Flavor, Orange Juice Powder, Magnesium Stearate, Stearic Acid, Gelatin, Stevia, Fumed Silica.
CompleteNate Chewable tablets are formulated to provide vitamin and mineral supplementation throughout pregnancy and during postnatal period, for both the lactating and non-lactating mother. It is also useful for improving nutritional status prior to conception.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Safety and effectiveness in elderly patients have not been established.
-
DRUG INTERACTIONS
CompleteNate Chewable Tablets is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
-
ADVERSE REACTIONS
Folic Acid: Allergic sensitizations have been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and may subside with continuation of therapy and physician encouragement. Although the absorption of iron is best when taken between meals, occasional G.I. disturbances may be controlled by giving CompleteNate Chewable shortly after meals.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
- OVERDOSAGE
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
-
STORAGE
Store at 20°-25°C (68°-77°F), relative humidity no higher than 65%; excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from moisture and excessive heat. Note that contact with moisture may produce surface discoloration of the tablet. Dispense in a tight, light-resistant container with a child resistant closure as defined by the USP.
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-CNC-00002-1 Rev. 12/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com

- PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
COMPLETENATE CHEWABLE
.beta.-carotene, ascorbic acid, sodium ascorbate, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, and ferrous fumarate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-014 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 1000 [iU] ascorbic acid (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ascorbic acid 48 mg sodium ascorbate (UNII: S033EH8359) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 72 mg cholecalciferol (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) cholecalciferol 400 [iU] .alpha.-tocopherol acetate, dl- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 11 [iU] thiamine mononitrate (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 2 mg riboflavin (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) riboflavin 3 mg niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) niacinamide 20 mg pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) pyridoxine Hydrochloride 10 mg folic acid (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) folic acid 1 mg cyanocobalamin (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) cyanocobalamin 12 ug ferrous fumarate (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 29 mg Inactive Ingredients Ingredient Name Strength sorbitol (UNII: 506T60A25R) sucrose (UNII: C151H8M554) magnesium stearate (UNII: 70097M6I30) stearic acid (UNII: 4ELV7Z65AP) gelatin (UNII: 2G86QN327L) stevia rebaudiuna leaf (UNII: 6TC6NN0876) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ORANGE OIL (UNII: AKN3KSD11B) ORANGE (UNII: 5EVU04N5QU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-014-90 90 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/01/2010 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color flavor imprint scoring 1 shape size (solid drugs) 12 mm Labeler - Trigen Laboratories, LLC (830479668)


