Label: NO PAIN MORE GAIN- menthol aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 69161-002-01 - Packager: Lugus Group LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
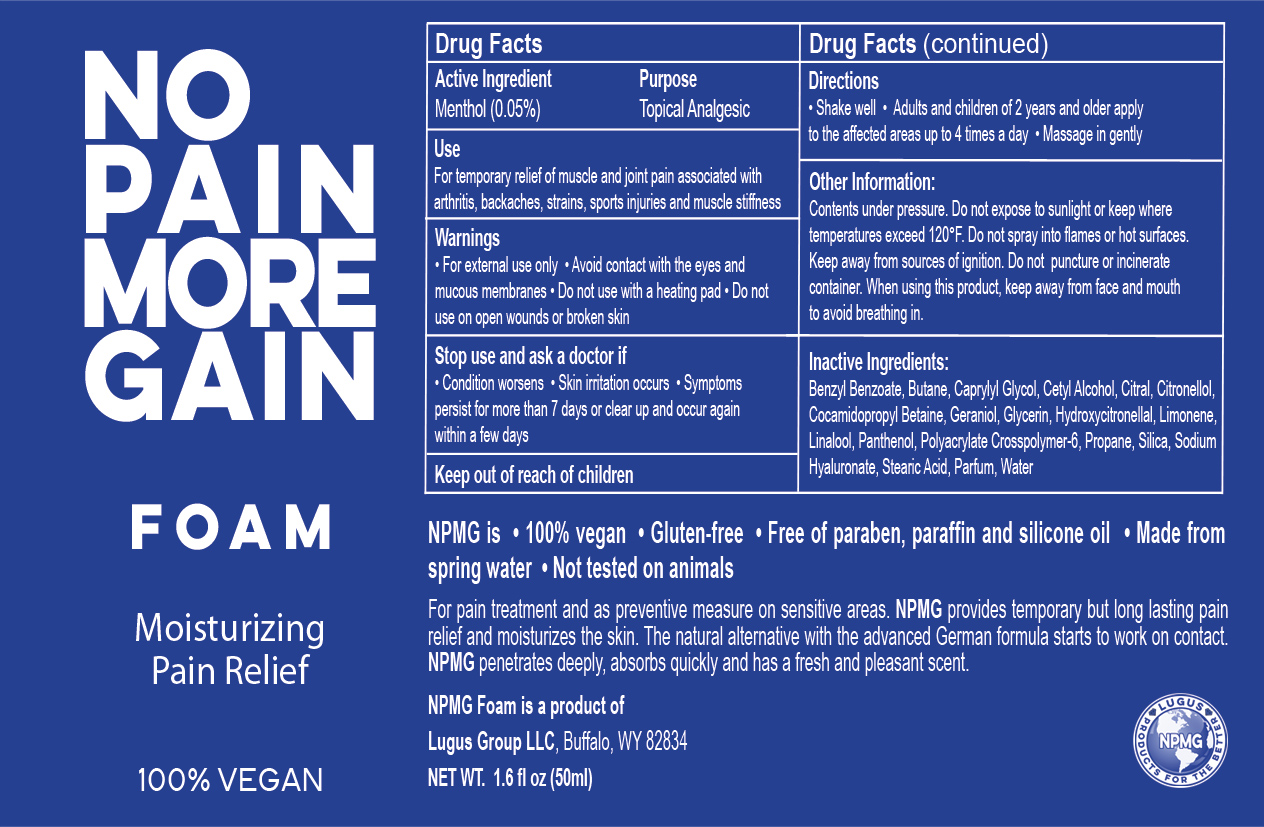
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
-
OTHER SAFETY INFORMATION
Other Information
Contents under pressure. Do not expose to sunlight or keep where temperatures exceed 120°F. Do not spray into flames or hot surfaces. Keep away from sources of ignition. Do not puncture or incinerate container. When using this product, keep away from face and mouth to avoid breathing in.
-
INDICATIONS & USAGE
100% Vegan
No Pain More Gain Foam
Moisturizing Pain Relief
NPMG is • 100% vegan • Gluten-free • Free of paraben, paraffin and silicone oil • Made from spring water • Not tested on animals
For pain treatment and as preventive measure on sensitive areas. NPMG provides temporary but long lasting pain relief and moisturizes the skin. The natural alternative with the advanced German formula starts to work on contact. NPMG penetrates deeply, absorbs quickly and has a fresh and pleasant scent.
NPMG Foam is a product of
Lugus Group LLC, Buffalo, WY 82834
NET WT 2.5 fl oz (75 ml)
- STOP USE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NO PAIN MORE GAIN
menthol aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69161-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 mg in 100 mg Inactive Ingredients Ingredient Name Strength BUTANE (UNII: 6LV4FOR43R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPANE (UNII: T75W9911L6) CETYL ALCOHOL (UNII: 936JST6JCN) PANTHENOL (UNII: WV9CM0O67Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) STEARIC ACID (UNII: 4ELV7Z65AP) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) BENZYL BENZOATE (UNII: N863NB338G) CITRAL (UNII: T7EU0O9VPP) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) GERANIOL (UNII: L837108USY) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+)- (UNII: F4VNO44C09) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69161-002-01 75 mg in 1 PACKAGE; Type 0: Not a Combination Product 08/29/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/29/2014 Labeler - Lugus Group LLC (079463507) Establishment Name Address ID/FEI Business Operations Lugus Group LLC 079463507 label(69161-002)