Label: FIRST AID DIRECT CHEWABLE ASPIRIN- aspirin tablet, chewable
- NDC Code(s): 42961-104-02
- Packager: Cintas Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy Allert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an (NSAID), which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- Do not use
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
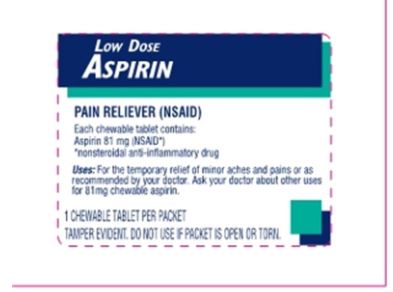
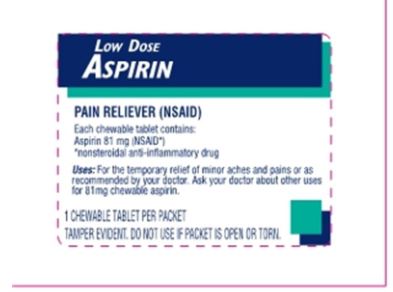
Principal Display Panel
LOW DOSE ASPIRIN
25 Chewable Tablets
1 Tablet per packet
Pain Reliever
Temporary relief of minor aches and pains or as recommended by your doctor
Compare active ingredient to Bayer® Chewable Aspirin
Manufactured for: fad first aid direct Mason, OH 45040


LOW DOSE ASPIRIN
PAIN RELIEVER (NSAID)
Each chewable tablet contains:
Aspirin 81mg (NSAID*)
*nonsteroidal anti-inflammatory drug
Uses: For the temporary relief of minor aches and pains or as recommended by your doctor. Ask your doctor about other uses for 81mg chewable aspirin.
1 CHEWABLE TABLET PER PACKET
TAMPER EVIDENT. DO NOT USE IF PACKET IS OPEN OR TORN.
-
INGREDIENTS AND APPEARANCE
FIRST AID DIRECT CHEWABLE ASPIRIN
aspirin tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DEXTRATES (UNII: G263MI44RU) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color orange Score no score Shape ROUND Size 8mm Flavor ORANGE Imprint Code AZ013 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-104-02 25 in 1 BOX 10/25/2021 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 10/25/2021 Labeler - Cintas Corp (056481716) Establishment Name Address ID/FEI Business Operations Allegiant Health 079501930 manufacture(42961-104)