Label: ADCIRCA- tadalafil tablet
- NDC Code(s): 66302-467-60
- Packager: United Therapeutics Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADCIRCA safely and effectively. See full prescribing information for ADCIRCA. ADCIRCA (tadalafil) tablets for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension - ADCIRCA® is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Studies establishing ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Pulmonary Arterial Hypertension - The recommended dose of ADCIRCA is 40 mg (two 20 mg tablets) taken once daily with or without food. Dividing the dose (40 mg) over the course of the day is ...
-
3 DOSAGE FORMS AND STRENGTHS
20 mg, orange, film-coated, almond-shaped tablets (not scored) debossed with “4467”.
-
4 CONTRAINDICATIONS
4.1 Concomitant Organic Nitrates - ADCIRCA is contraindicated in patients who are using any form of organic nitrate, either regularly or intermittently. Do not use nitrates within 48 hours of ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension - ADCIRCA has vasodilatory properties that may result in transient decreases in blood pressure. Prior to prescribing ADCIRCA, carefully consider whether patients with underlying ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling: Hypotension [see Warnings and Precautions (5.1)] Visual Loss [see Warnings and Precautions (5.3) and Patient ...
-
7 DRUG INTERACTIONS
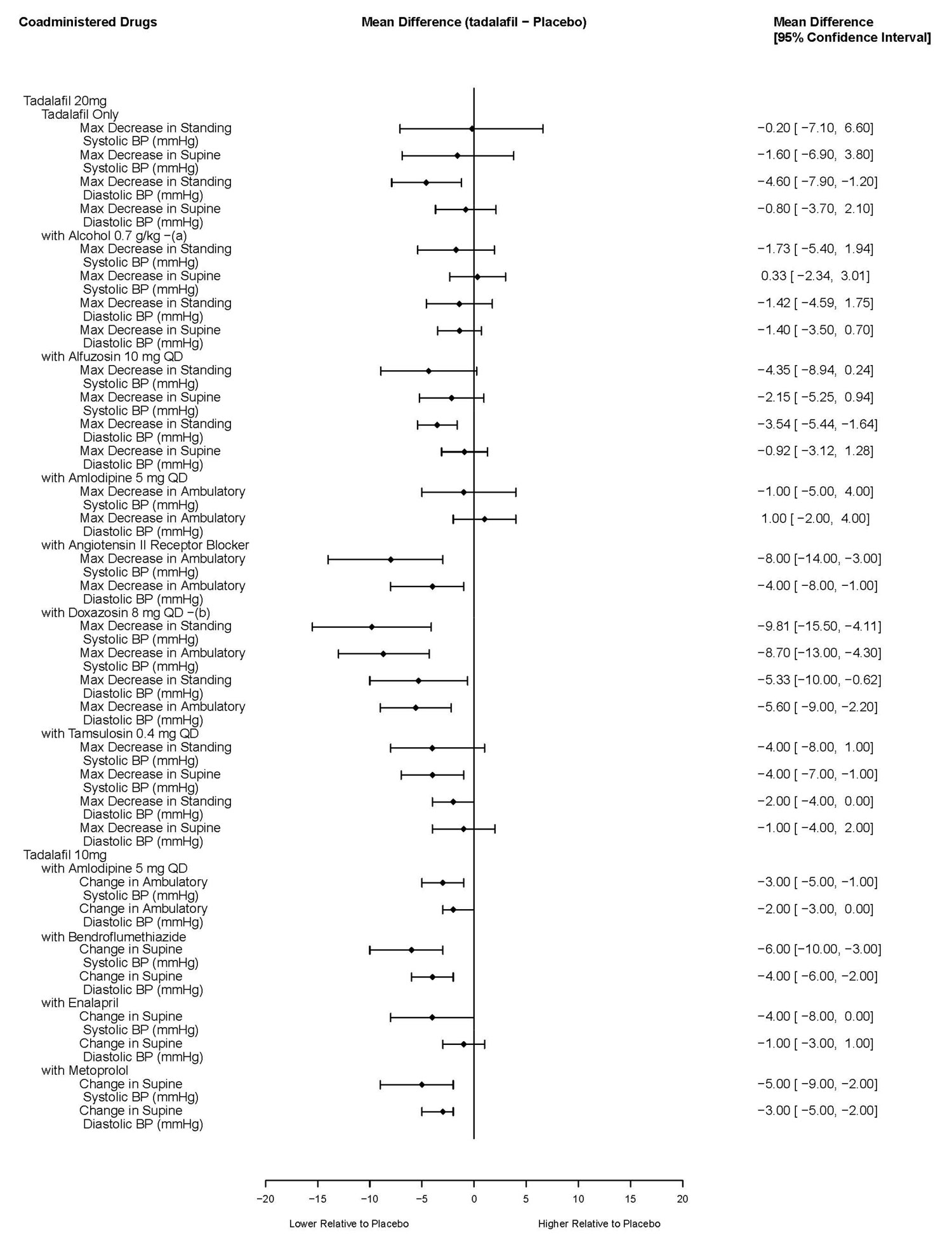
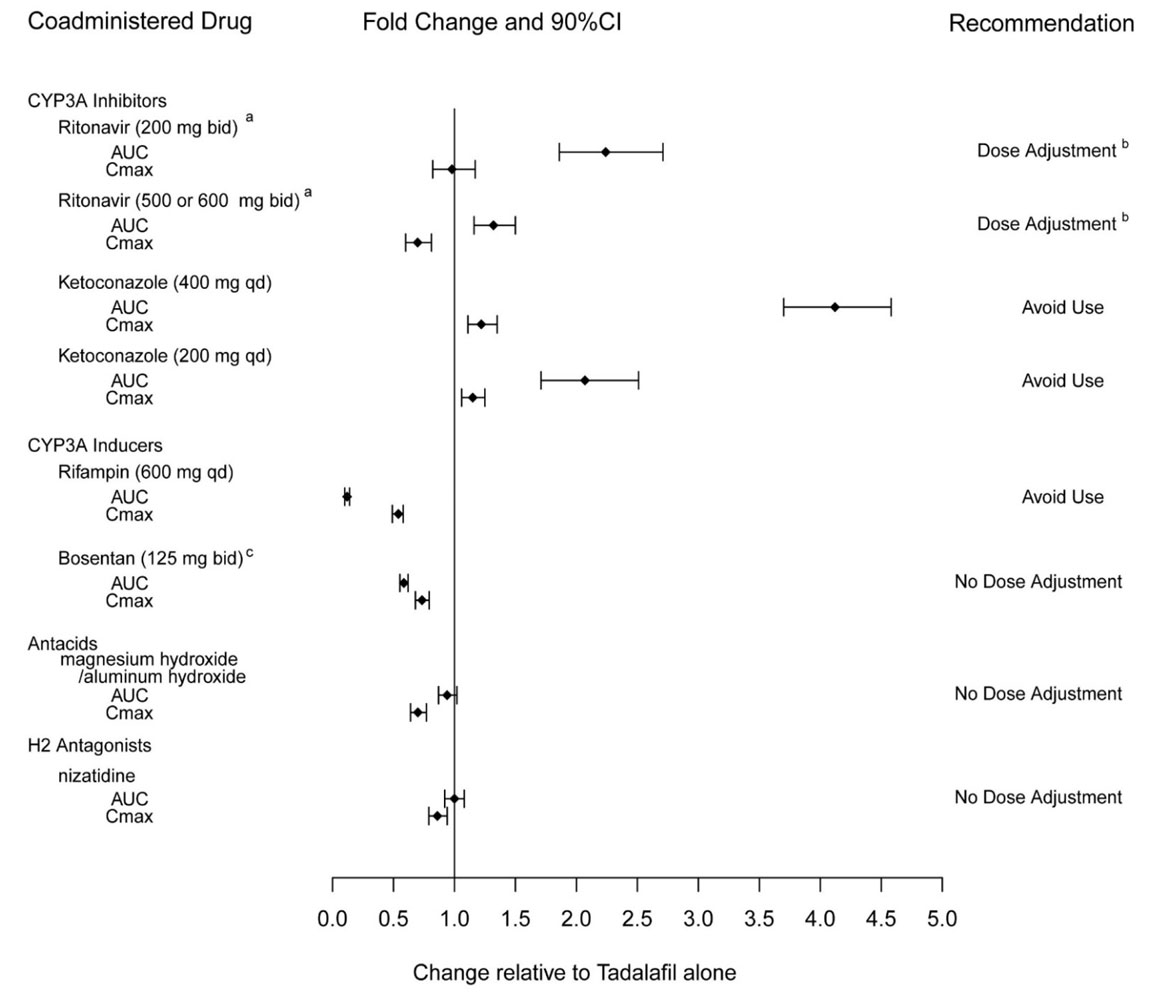
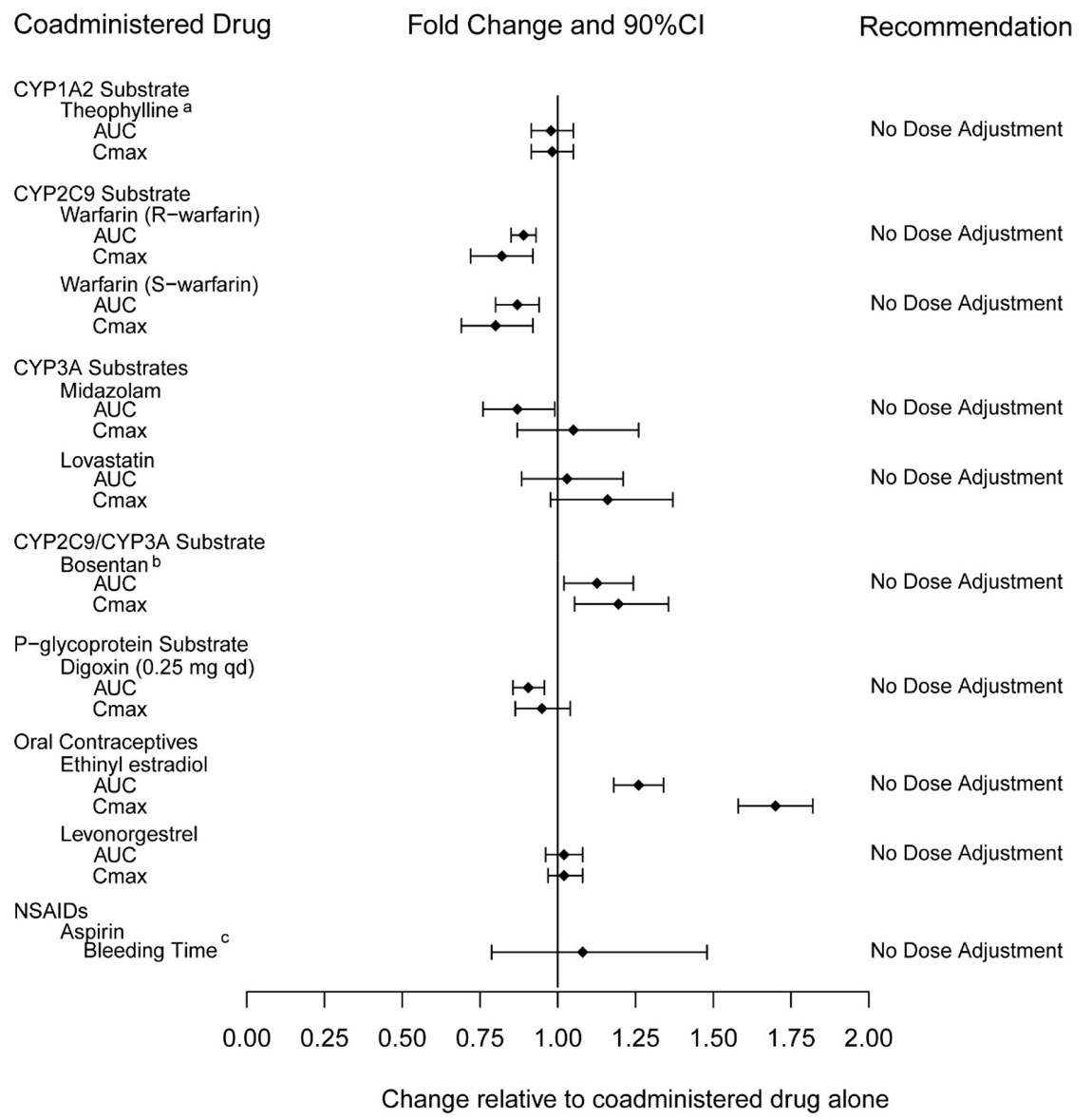
7.1 Nitrates - Administration of nitrates within 48 hours after the last dose of ADCIRCA is contraindicated [see Contraindications (4.1)]. 7.2 Alpha-Blockers - PDE5 inhibitors, including ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Limited data from case series with tadalafil use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse ...
-
10 OVERDOSAGE
Single doses up to 500 mg have been given to healthy male subjects, and multiple daily doses up to 100 mg have been given to male patients with erectile dysfunction. Adverse reactions were similar ...
-
11 DESCRIPTION
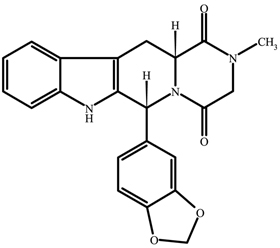
ADCIRCA (tadalafil), an oral treatment for pulmonary arterial hypertension, is a selective inhibitor of cyclic guanosine monophosphate (cGMP)–specific phosphodiesterase type 5 (PDE5). Tadalafil ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Tadalafil is an inhibitor of phosphodiesterase type 5 (PDE5), the enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). Pulmonary arterial ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis — Tadalafil was not carcinogenic to rats or mice when administered daily for 2 years at doses up to 400 mg/kg/day ...
-
14 CLINICAL STUDIES
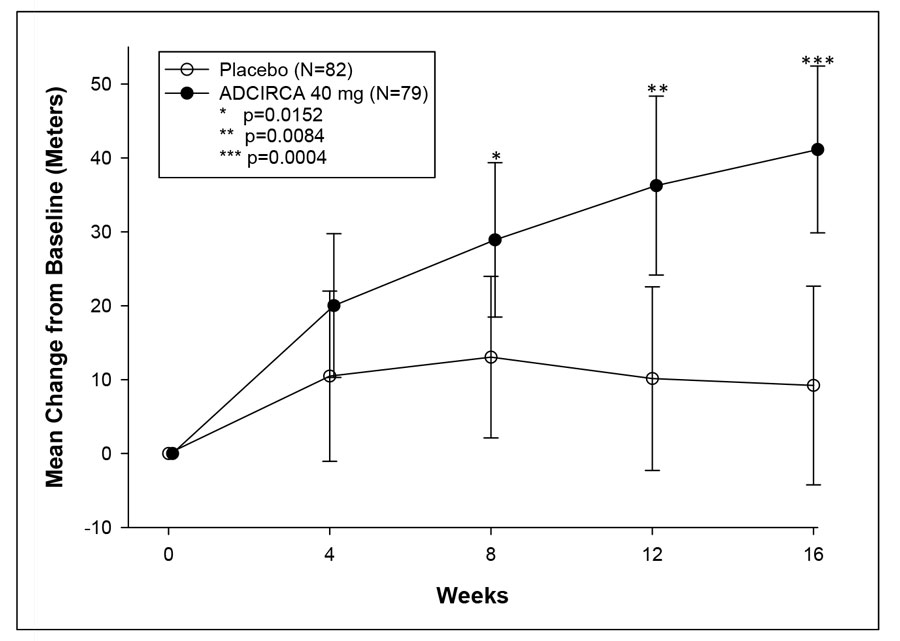
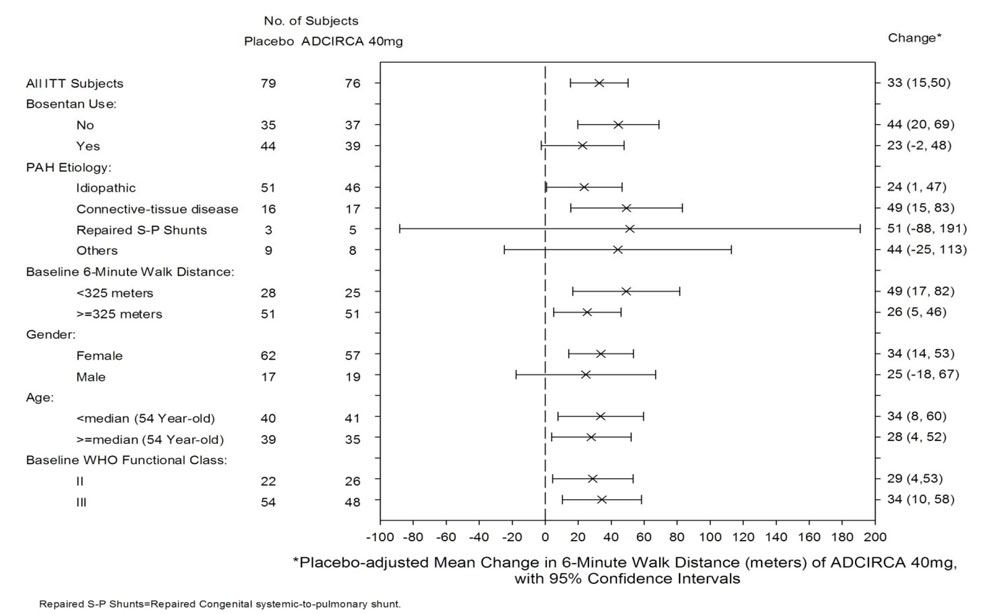
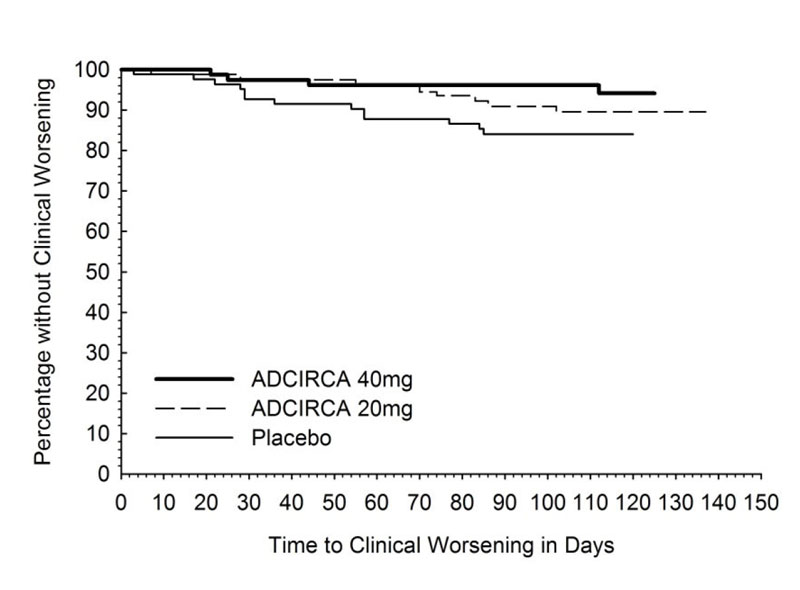
14.1 ADCIRCA for Pulmonary Arterial Hypertension - A randomized, double-blind, 16 week placebo-controlled study was conducted in 405 patients with pulmonary arterial hypertension, defined as a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - ADCIRCA (tadalafil) is supplied as follows: 20 mg orange, film–coated, almond–shaped tablets (not scored), debossed with “4467” Bottles of 60 NDC ...
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information) Inform patients of contraindication of ADCIRCA with any use of organic nitrates or GC stimulators. Inform patients that tadalafil is ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Eli Lilly and Company, Indianapolis, IN 46285, USA - Marketed by: United Therapeutics Corporation - Copyright © 2009, 2020, Eli Lilly and Company. All rights ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ADCIRCA® (Ad-sur-kuh) (tadalafil) tablets - Read this patient information before you start taking ADCIRCA and each time you get a refill. There may be new information. This ...
-
PACKAGE LABEL – ADCIRCA 20 mg60 tablets - NDC 66302-467-60 - Rx only - TA 4467 - adcirca® tadalafil tablets - 20 mg 4467 - United Therapeutics Corporation - Lilly - Same active ingredient as ...
-
INGREDIENTS AND APPEARANCEProduct Information