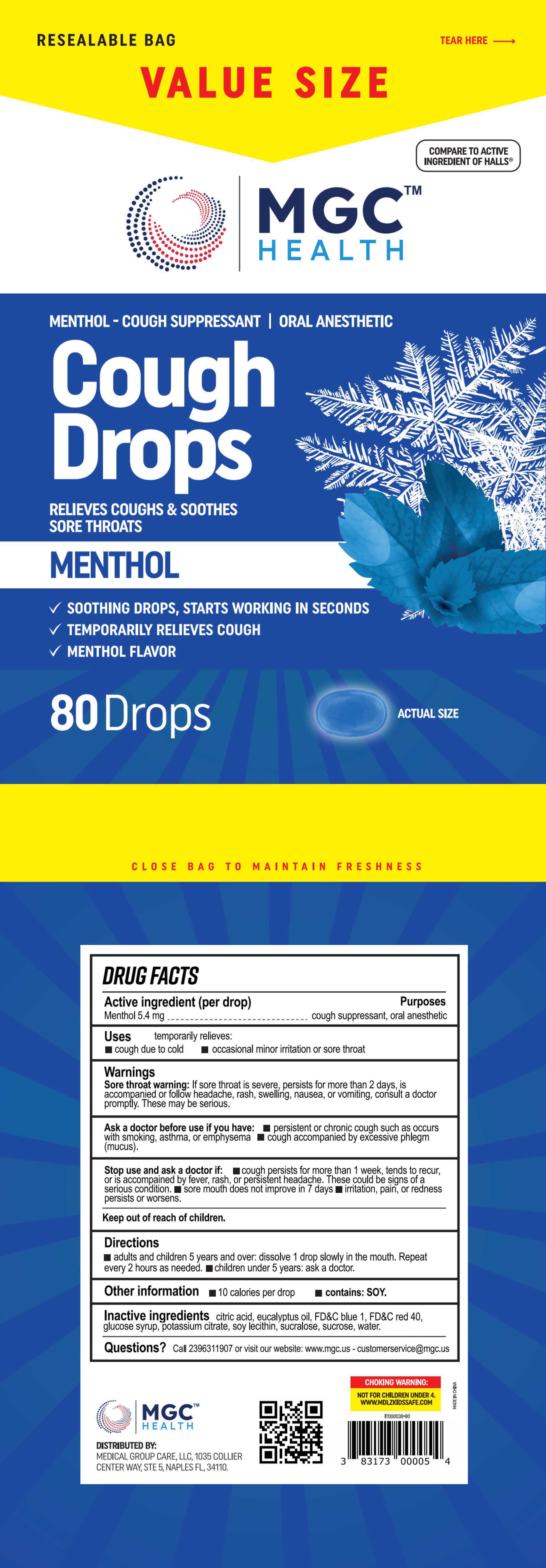
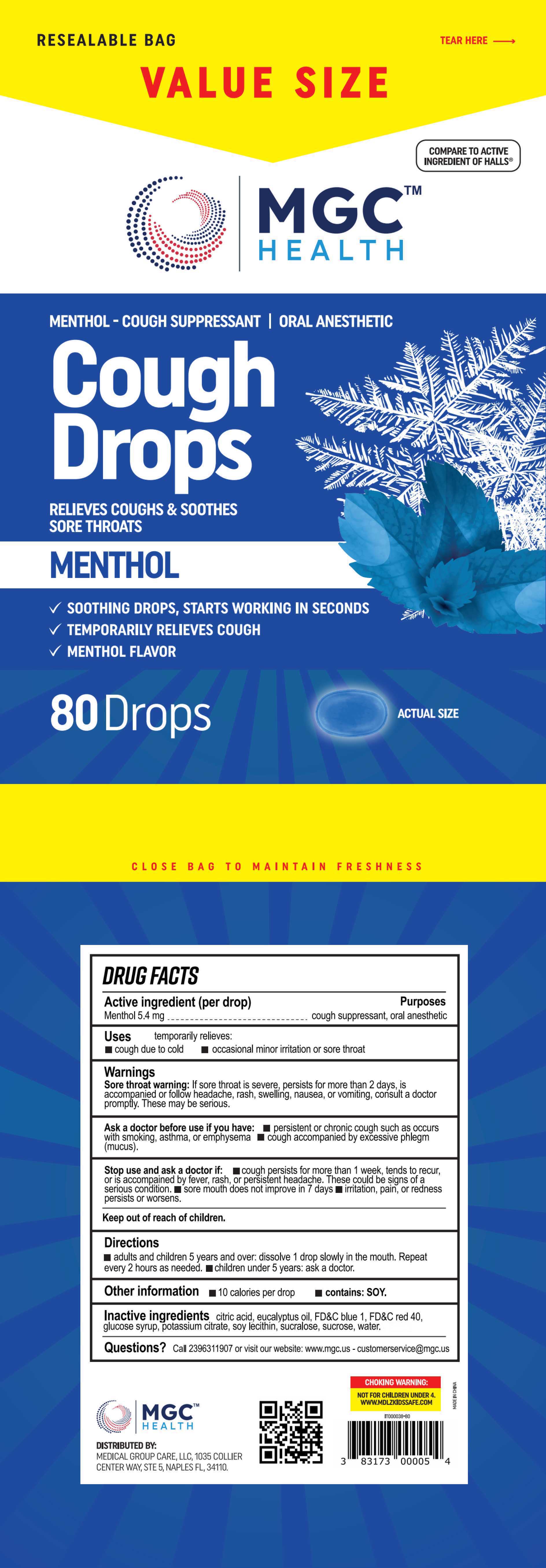
Label: COUGH DROPS- menthol tablet, orally disintegrating
- NDC Code(s): 80404-501-01, 80404-501-02, 80404-501-03, 80404-501-04
- Packager: Xinsanyang Pharmaceutical (Xiamen) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DO NOT USE
Ask a doctor before use if you have:persistent or chronic cough such as occurs with smoking, asthma, or emphysema
cough accompanied by excessive phlegm (mucus).
Stop use and ask a doctor if: cough persists for more than 1 week, tends to recur,
or is accompained by fever, rash, or persistent headache. These could be signs of a
serious condition. sore mouth does not improve in 7 daysirritation, pain, or redness persists or worsens.
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COUGH DROPS
menthol tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80404-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.4 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EUCALYPTUS OIL (UNII: 2R04ONI662) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CORN SYRUP (UNII: 9G5L16BK6N) POTASSIUM CITRATE (UNII: EE90ONI6FF) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) FD&C RED NO. 40 (UNII: WZB9127XOA) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) Product Characteristics Color blue Score score with uneven pieces Shape OVAL Size 20mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80404-501-01 25 in 1 BAG; Type 0: Not a Combination Product 06/29/2023 2 NDC:80404-501-02 30 in 1 BAG; Type 0: Not a Combination Product 06/29/2023 3 NDC:80404-501-03 70 in 1 BAG; Type 0: Not a Combination Product 06/29/2023 4 NDC:80404-501-04 80 in 1 BAG; Type 0: Not a Combination Product 06/29/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/29/2023 Labeler - Xinsanyang Pharmaceutical (Xiamen) Co., Ltd. (546457554) Establishment Name Address ID/FEI Business Operations Xinsanyang Pharmaceutical (Xiamen) Co., Ltd. 546457554 manufacture(80404-501)