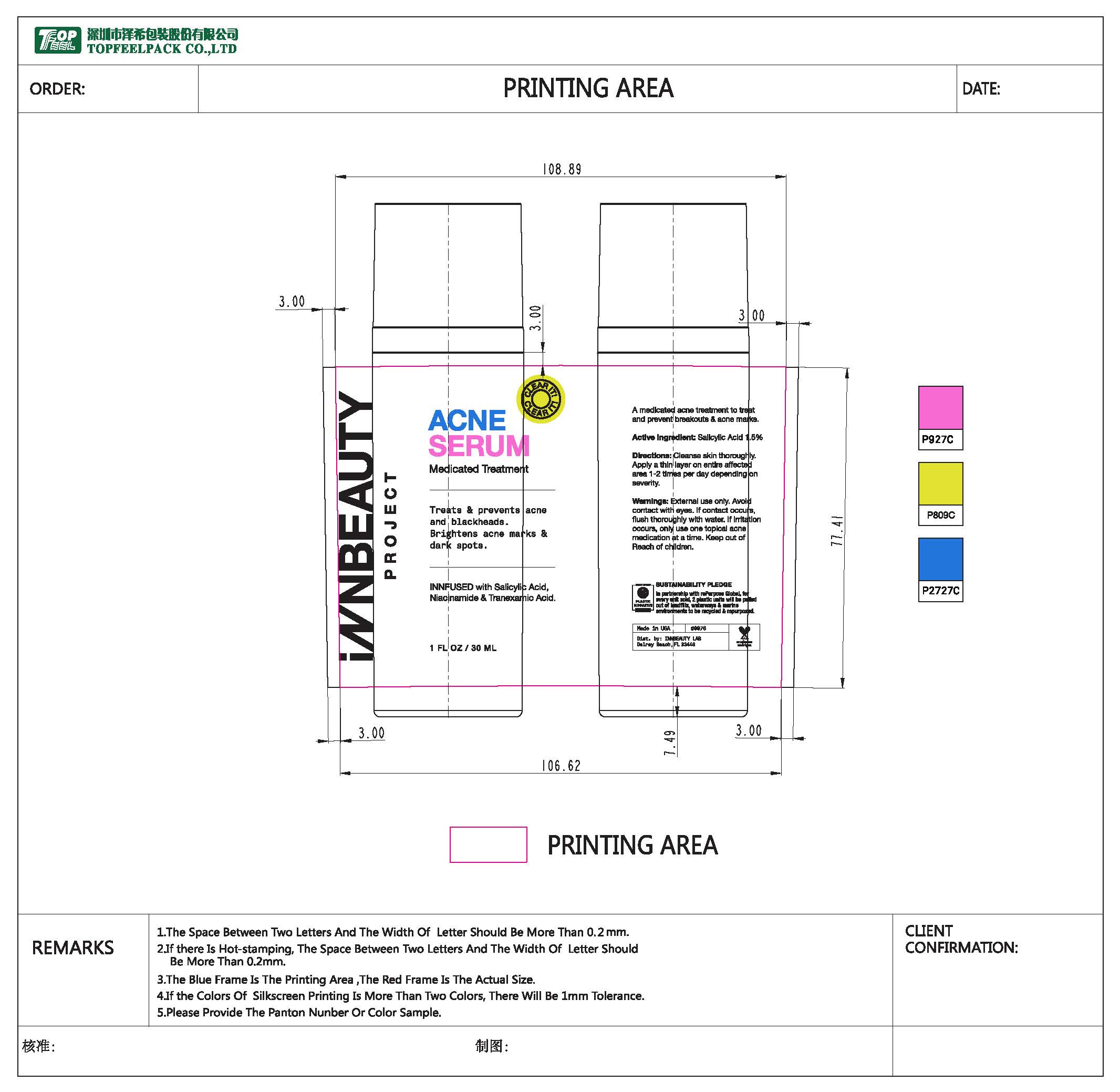
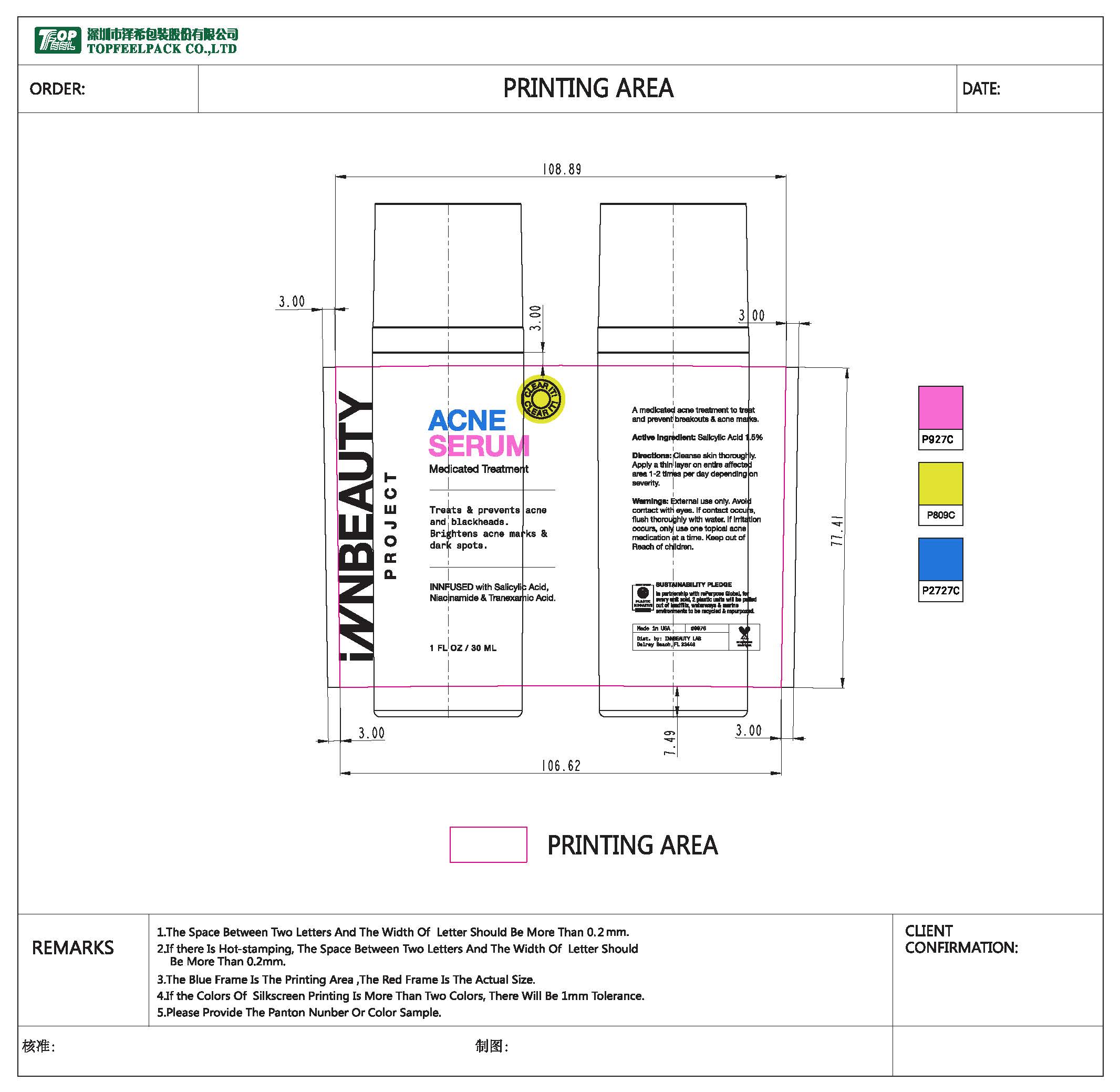
Label: CREAM- salicylic acid lotion
- NDC Code(s): 61354-099-01
- Packager: Oxygen Development LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using use only
- Keep out of reach of children
- Directions
- Other information
-
Inactive Ingredient
Water/Aqua, Propanediol, Glycerin, Caprylic/Capric Triglyceride, Dimethyl Isosorbide, Lactobacillus Ferment, Coconut Alkanes, Coco-Caprylate, Niacinamide, Candelilla/Jojoba/Rice Bran Polyglyceryl-3 Esters, Tranexamic Acid, Hexyl Laurate, Glycine Soja Soybean, C15-19 Alkane, Potassium Azeloyl Diglycinate, Hydrogenated Polydecene, Glyceryl Stearate, Ceramide NP, Ceramide AP, Bakuchiol, Fucus Spiralis Extract, Rhodomyrtus Tomentosa Fruit Extract, Tasmannia Lanceolata Fruit/Leaf Extract, Acetyl Glucosamine, Hydrolyzed Sodium Hyaluronate, Zinc Sulfate, Melia Azadirachta Leaf Extract, Centella Asiatica Leaf Extract, Silybum Marianum Seed Extract, Vitex Agnus - Castus Extra, Helianthus Annuus (Sunflower) Extract, Oryza Sativa (Rice) Bran Extract, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, Allantoin, Panthenol, 1,2 - Hexanediol, Sodium Stearoyl Lactylate, Citric Acid, Xanthan Gum, Polyglyceryl - 3 Diisostearate, Polyacrylate Crosspolymer - 6, Coco - Caprylate/Caprate, Caprylhydroxamic Acid, Sodium Phytate, Tocopherol, Sodium Benzoate, Potassium Sorbate, Biosaccaharide Gum - 1, Phenoxyethanol, Sodium Anisate.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CREAM
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61354-099 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) 3 mg in 100 mg CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) 2.5 mg in 100 mg C15-19 ALKANE (UNII: CI87N1IM01) 1.2 mg in 100 mg DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) 4 mg in 100 mg COCONUT ALKANES (UNII: 1E5KJY107T) 3.76 mg in 100 mg MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 5.5 mg in 100 mg COCO-CAPRYLATE (UNII: 4828G836N6) 3.5 mg in 100 mg LIMOSILACTOBACILLUS FERMENTUM (UNII: 2C1F12C6AP) 3.97 mg in 100 mg TRANEXAMIC ACID (UNII: 6T84R30KC1) 2 mg in 100 mg BAKUCHIOL (UNII: OT12HJU3AR) 0.5 mg in 100 mg POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) 1 mg in 100 mg GLYCERIN (UNII: PDC6A3C0OX) 6.07 mg in 100 mg DOCOSANOL (UNII: 9G1OE216XY) 2.5 mg in 100 mg SOYBEAN OIL (UNII: 241ATL177A) 1.3 mg in 100 mg 1,2-HEXANEDIOL (UNII: TR046Y3K1G) 0.75 mg in 100 mg SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) 0.5 mg in 100 mg HEXYL LAURATE (UNII: 4CG9F9W01Q) 1.5 mg in 100 mg GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) 1 mg in 100 mg HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) 1 mg in 100 mg CANDELILLA WAX (UNII: WL0328HX19) 2.5 mg in 100 mg WATER (UNII: 059QF0KO0R) 38.78 mg in 100 mg PROPANEDIOL (UNII: 5965N8W85T) 7.47 mg in 100 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61354-099-01 1 in 1 CARTON 06/26/2023 1 100 mg in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 06/26/2023 Labeler - Oxygen Development LLC (137098492) Establishment Name Address ID/FEI Business Operations Oxygen Development LLC 137098492 manufacture(61354-099)