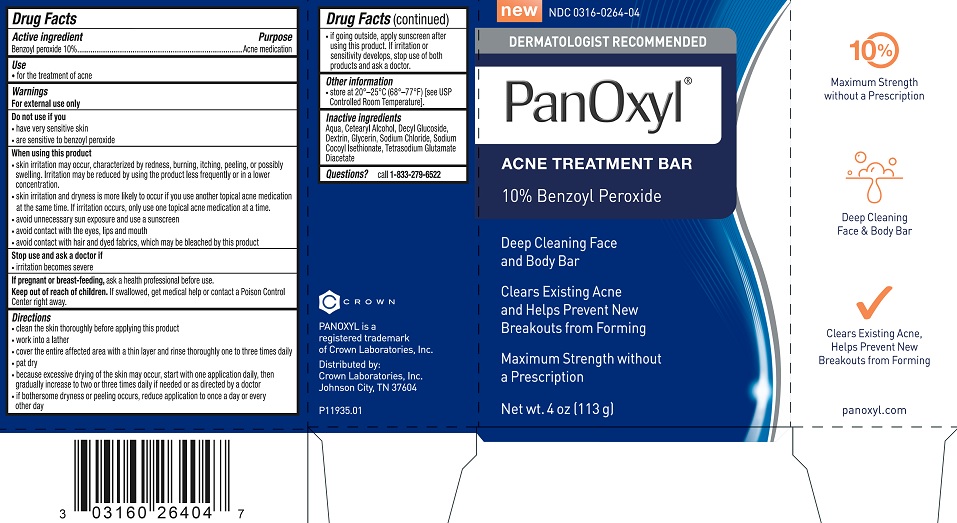
Label: PANOXYL- benzoyl peroxide soap
- NDC Code(s): 0316-0264-01, 0316-0264-04
- Packager: Crown Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
-
Directions
- clean the skin thoroughly before applying this product
- work into a lather
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- pat dry
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other information
- Inactive ingredients
- Questions?
- Panoxyl Acne Treatmet Bar Carton
-
INGREDIENTS AND APPEARANCE
PANOXYL
benzoyl peroxide soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0316-0264 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 12.6 g in 113 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) ICODEXTRIN (UNII: 2NX48Z0A9G) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0316-0264-04 113 g in 1 CARTON; Type 0: Not a Combination Product 07/01/2023 2 NDC:0316-0264-01 43 g in 1 CARTON; Type 0: Not a Combination Product 02/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/01/2023 Labeler - Crown Laboratories (079035945)