Label: NO7 LABORATORIES ACNE TREATMENT- salicylic acid liquid
- NDC Code(s): 11489-176-01
- Packager: BCM Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Dosage
- Uses
- Warnings
- Keep out of reach of children
-
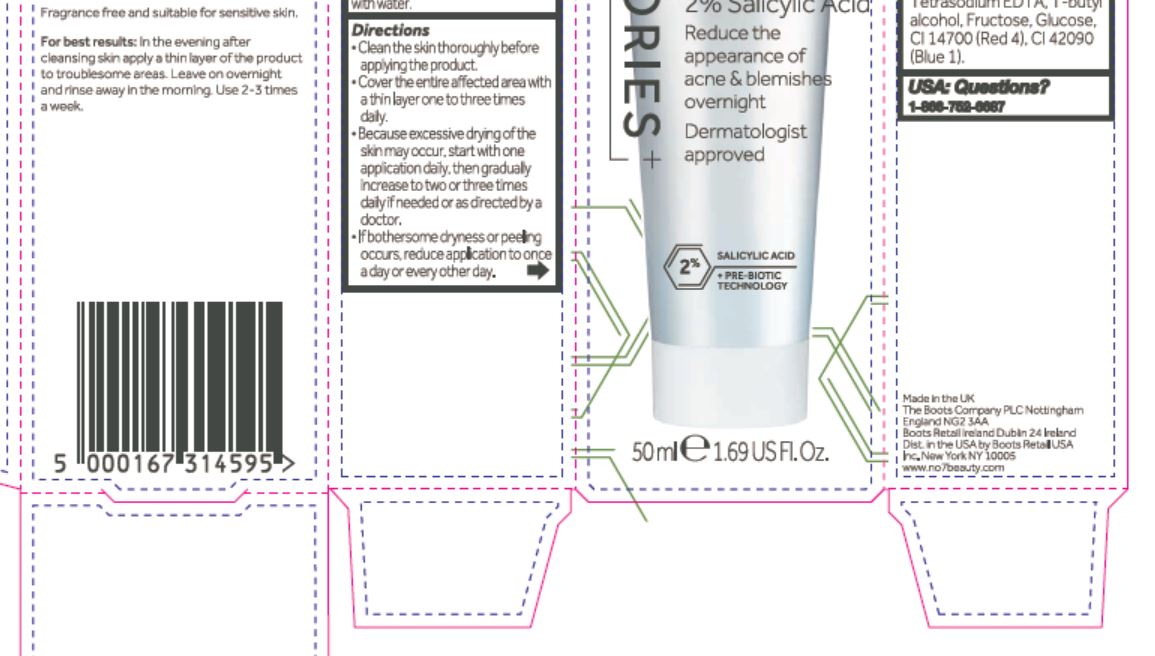
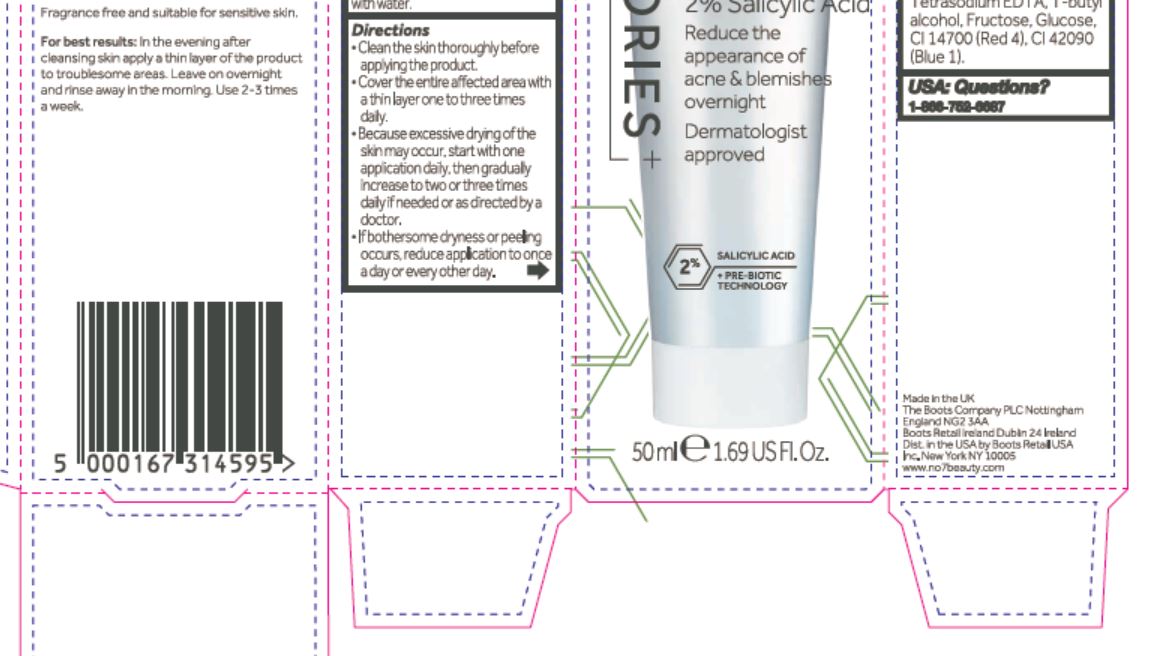
Directions
Directions
Clean the skin thoroughly before applying the product.
Cover the entire affected area with a thin layer one to three times daily.
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
Inactive ingredients
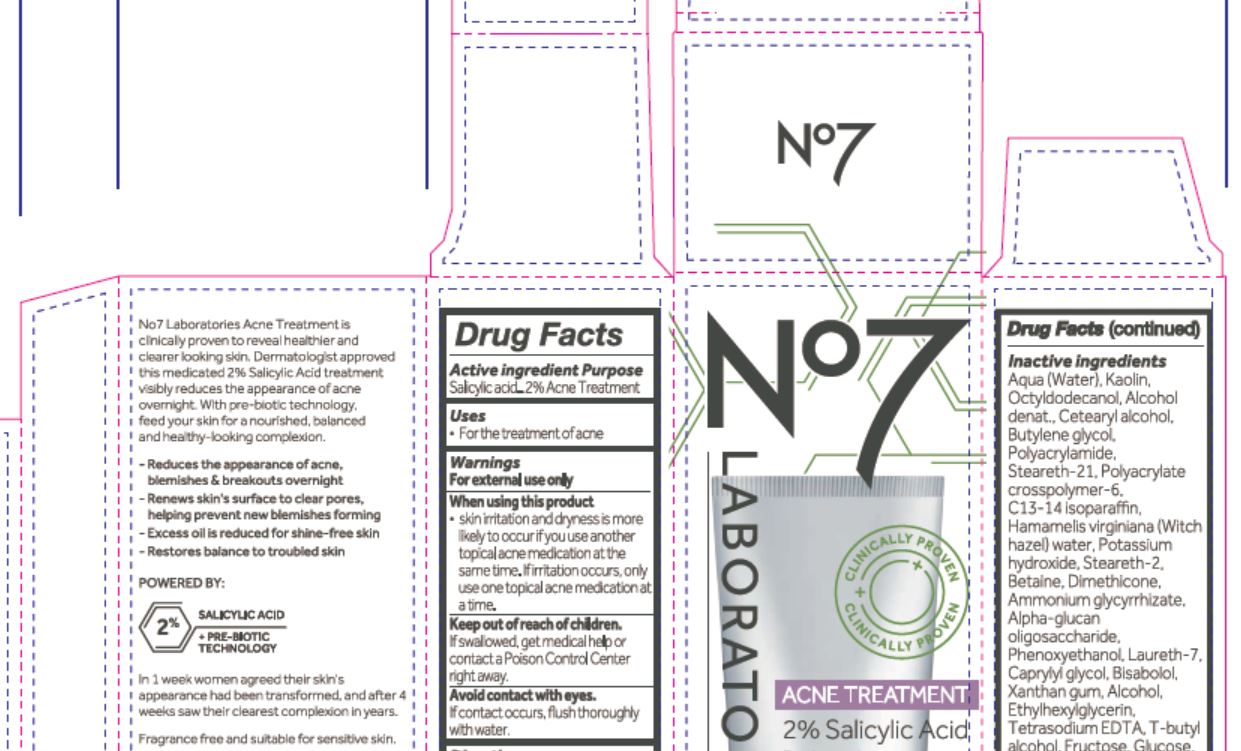
Aqua (Water), Kaolin, Octyldodecanol, Alcohol denat., Cetearyl alcohol, Butylene glycol, Polyacrylamide, Steareth-21, Polyacrylate crosspolymer-6, C13-14 isoparaffin, Hamamelis virginiana (Witch hazel) water, Potassium hydroxide, Steareth-2, betaine, Dimethicon, Ammonium glycyrrhizate, Alpha-glucan oligosaccharide, Phenoxyethanol, Laureth-7, Caprylyl glycol, Bisabolol, Xanthan gum, Alcohol, Exylhexylglycerin, Tetrasodium EDTA, T-butyl alcohol, Fructose, Glucose, CI 14700 (Red 4), CI 42090 (Blue 1).
- INFORMATION FOR PATIENTS
- Questions
-
Description
No7
LABORATORIES
CLINICALLY PROVEN
CLINICALLY PROVEN
ACNE TREATMENT
2% Salicylic Acid
Reduce the appearance of acne & blemishes overnight
Dermatologist approved
2% SALICYLIC ACID
+ PRE-BIOTIC TECHNOLOGY
50 ml e 1.69 US Fl. Oz.
No7 Laboratories Acne Treatment is clinically proven to receal healthier and clearer looking skin. Dermatologist approved this medicated 2% Salicylic Acid treatment visibly reduces the appearance of acne overnight. With pre-biotic technology, feed your skin for a nourished, blanaced and health-looking complexion.
- Reduces the apperance of acne, blemishes & breakouts overnight
- Renews skin's surface to clear pores, helping to prevent new blemishes forming
- Excess oil is reduced for shine-free skin
- Restores balance to troubled skin
POWERED BY:
2% SALICYLIC ACID
+ PRE-BIOTIC TECHNOLOGY
In 1 week women agreed their skin's appearance had been transformed, and after 4 weeks saw their clearest complexion in years.
Fragrance free and suitable for sensitive skin.
For best results: In the evening after cleansing skin apply a thin layer of the product to troublesome area. Leav on overnight and rinse away in the morning. Use 2-3 times a week.
- Carton
-
INGREDIENTS AND APPEARANCE
NO7 LABORATORIES ACNE TREATMENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11489-176 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 50 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) KAOLIN (UNII: 24H4NWX5CO) OCTYLDODECANOL (UNII: 461N1O614Y) ALCOHOL (UNII: 3K9958V90M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) STEARETH-21 (UNII: 53J3F32P58) TRIMETHYLOLPROPANE TRIACRYLATE (UNII: 4B67KGL96S) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) HAMAMELIS VIRGINIANA LEAF WATER (UNII: 8FP93ED6H2) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) STEARETH-2 (UNII: V56DFE46J5) BETAINE (UNII: 3SCV180C9W) DIMETHICONE (UNII: 92RU3N3Y1O) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) .ALPHA.-GLUCAN OLIGOSACCHARIDE (UNII: S95658MI3W) PHENOXYETHANOL (UNII: HIE492ZZ3T) LAURETH-7 (UNII: Z95S6G8201) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BISABOLOL OXIDE A (UNII: 16AE65F94Y) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) FRUCTOSE (UNII: 6YSS42VSEV) DEXTROSE (UNII: IY9XDZ35W2) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11489-176-01 1 in 1 CARTON 11/04/2020 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 11/04/2020 Labeler - BCM Ltd (230780322) Registrant - The Boots Company PLC (218622660) Establishment Name Address ID/FEI Business Operations BCM Ltd 230780322 manufacture(11489-176) , analysis(11489-176)