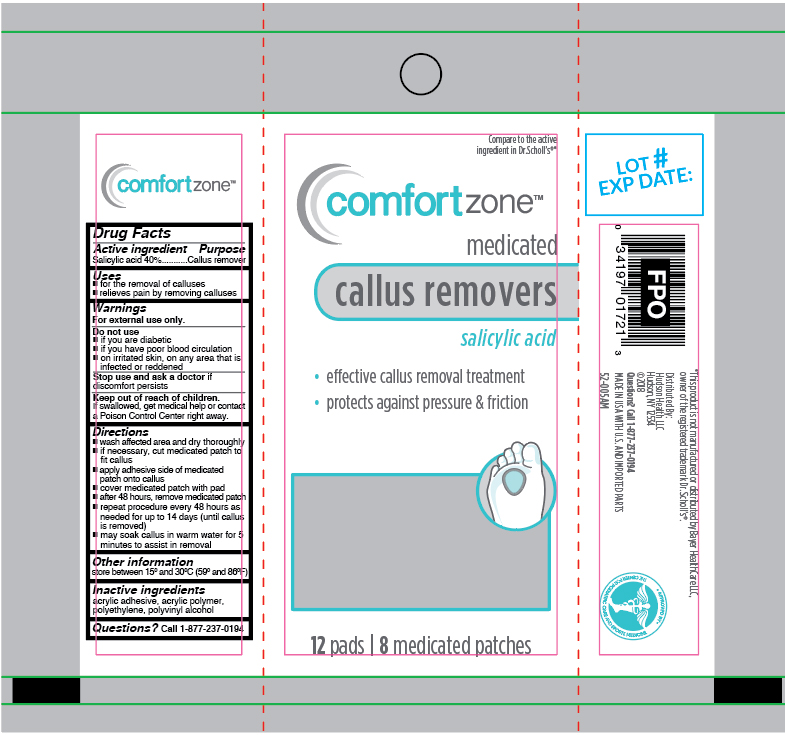
Label: SALICYLIC ACID- medicated callus removers patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 72446-001-08 - Packager: Hudson Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- wash affected area and dry thoroughlyif necessary, cut medicated patch to fit callus
- apply adhesive side down of medicated patch onto callus
- cover medicated patch with pad
- after 48 hours, remove medicated patch
- repeat procedure every 48 hours as needed for up to 14 days (until callus is removed)
- may soak callus in warm water for 5 minutes to assist in removal
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
medicated callus removers patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72446-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 mg in 8 Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) VINYL ACETATE (UNII: L9MK238N77) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72446-001-08 8 in 1 PACKAGE; Type 0: Not a Combination Product 08/31/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358F 08/31/2018 Labeler - Hudson Health LLC (081276171)