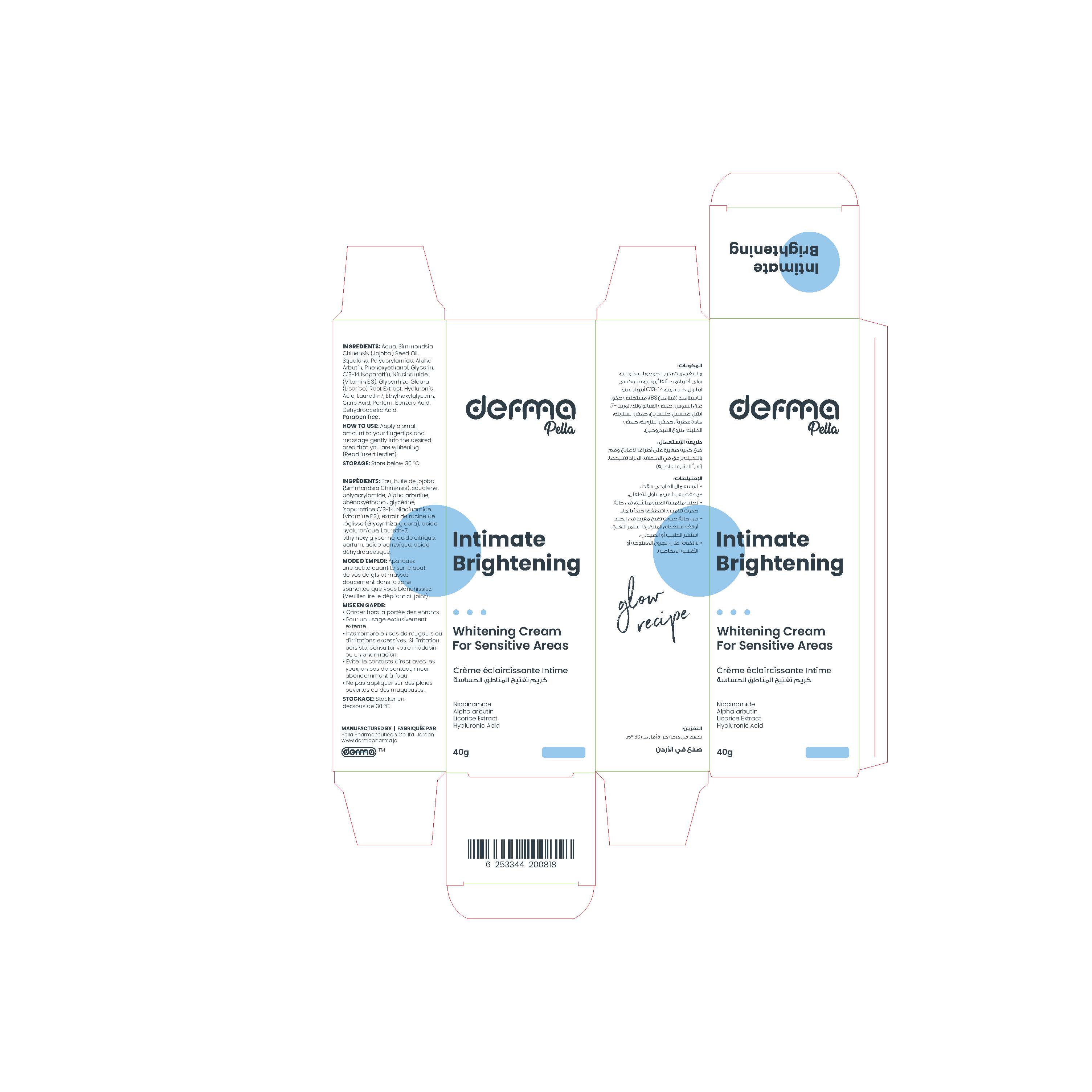
Label: DERMA PELLA INTIMATE BRIGHTENING- alpha arbutin cream
- NDC Code(s): 82160-818-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Form and Presentation
- Active Ingredient
- Inactive Ingredients
- Purpose
-
Properties
This product is formulated for use on all skin types and applicable to sensitive, intimate areas.
- Lightens, protects and rejuvenate the skin.
- Brings back the elasticity of the skin.
- Moisture the delicate skin.
- Soothes the skin in a pleasure way.
- Leaves a long-lasting refreshing aroma.Paraben free
- Indication
- Precautions
-
Warnings
- For external use only.
- Over application will not increase results.
- If you have a sensitive skin test in a small area in the underarm before using on all your intimate areas.
- Discontinue if excessive redness or irritation occurs. If irritation persists, consult with a physician or pharmacist.
- Avoid direct contact with eyes, if contact occurs, rinse thoroughly with water.
- Do not apply to open wounds or mucous membranes. - Contraindications
- Side effects
-
Dosage and Administration
Cleanse area with soap and water, and then gently exfoliate it with a loofah or sponge. This helps to remove any dead skin at the surface and allow the product to contact the fresh area that you want to whiten. Rinse the area and pat dry. Apply a small amount to fingertips and massage gently into the desired area that you are whitening. Allow time for it to absorb. After a few minutes, massage any excess cream into the area. Only use enough cream to apply a thin layer. Apply twice daily for maximum effectiveness.
- Storage Conditions
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
DERMA PELLA INTIMATE BRIGHTENING
alpha arbutin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-818 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPHA-ARBUTIN (UNII: 72VUP07IT5) (ALPHA-ARBUTIN - UNII:72VUP07IT5) ALPHA-ARBUTIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength JOJOBA OIL (UNII: 724GKU717M) SQUALENE (UNII: 7QWM220FJH) GLYCERIN (UNII: PDC6A3C0OX) LAURETH-7 (UNII: Z95S6G8201) NIACINAMIDE (UNII: 25X51I8RD4) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) WATER (UNII: 059QF0KO0R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-818-01 1 in 1 CARTON 05/09/2023 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/09/2023 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)