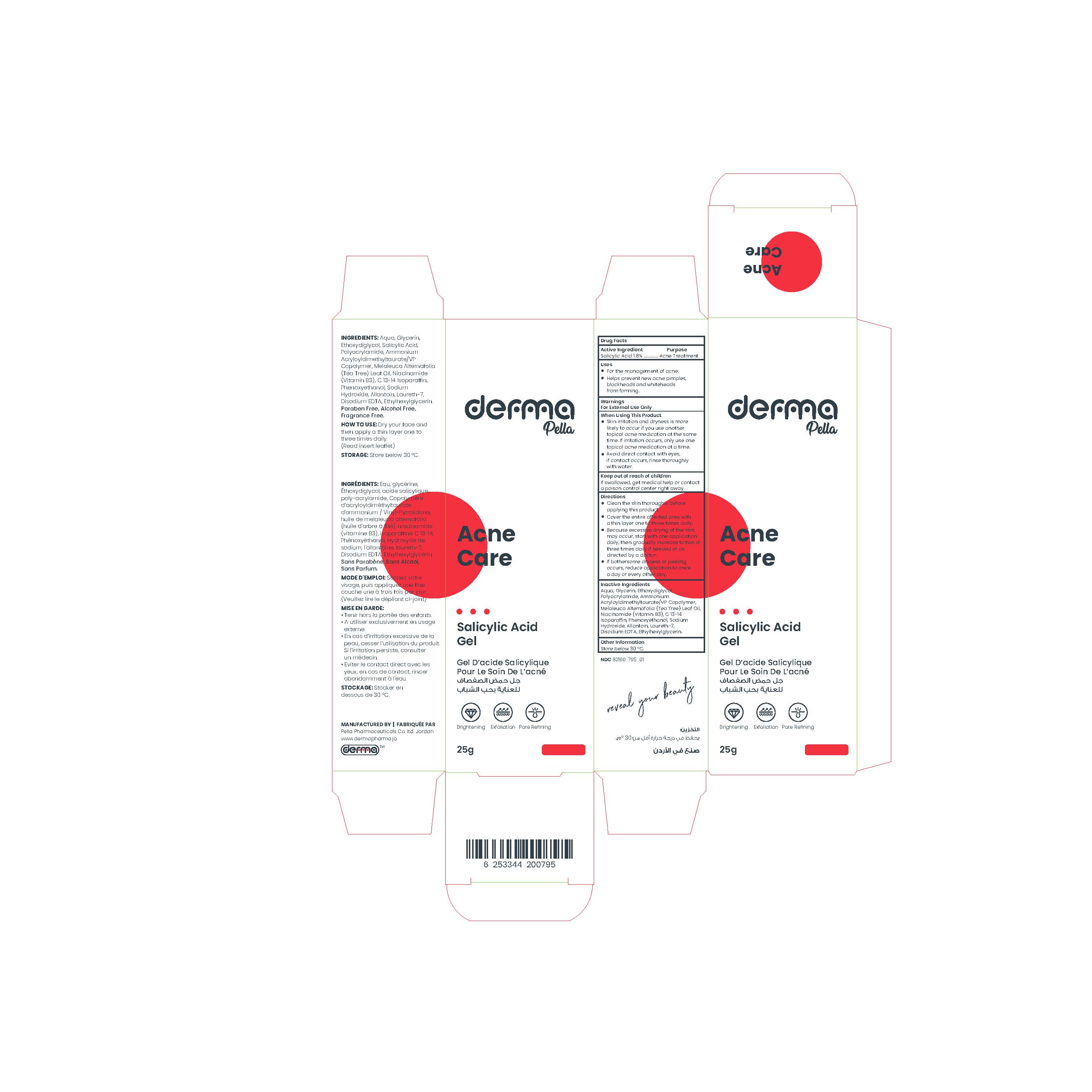
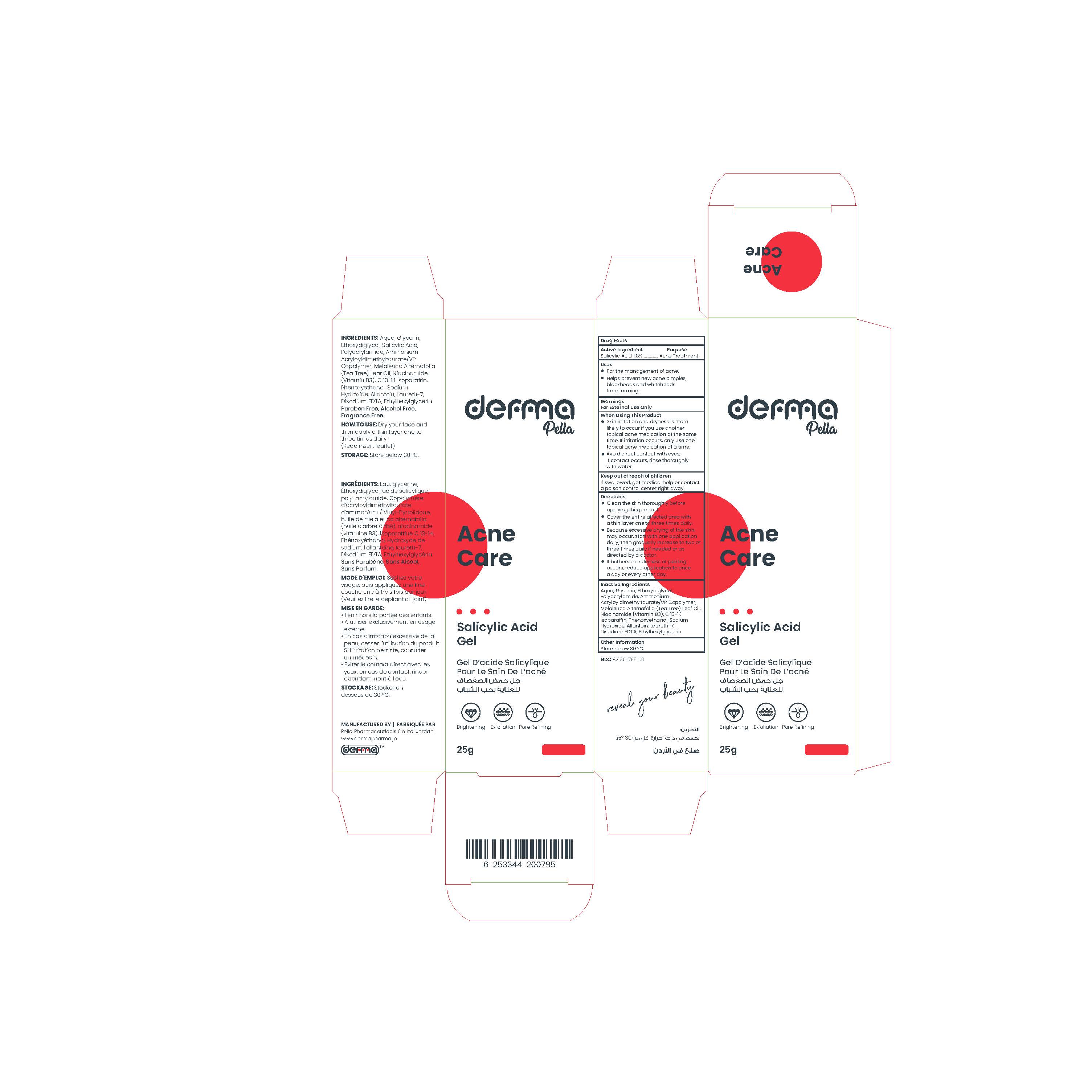
Label: DERMA PELLA ACNE CARE- salicylic acid gel
- NDC Code(s): 82160-795-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Form and Presentation
- Active Ingredient
- Inactive Ingredients
- Purpose
-
Properties
A lightweight, water-based alcohol-free gel.
This product contains a beta hydroxyl acid (BHA) which is a salicylic acid that clears out clogged pores, reduces blemishes, and helps to minimize blackheads and breakouts. It dissolves excess sebum, making it suitable for acne-prone skin.
And also, it contains Tea tree oil which has soothing properties and improves the appearance of marks.
In addition to Niacinamide (Vitamin B3) which works best to soothe and lighten skin, visibly minimize pores, and improve uneven skin tone, reduce dark spots and acne spots.
It contains Allantoin which is an effective moisturizing ingredient used for it is gentle and non-irritating qualities.
Paraben free, Alcohol free, Fragrance free. - Indications
- Precautions
- Warnings
- Contraindications
- Side Effects
-
Dosage and Administration
- Cleanse skin thoroughly before applying product.
- Dry your face and then apply a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, and then gradually increase to two or three times daily, if needed.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day. - Storage Conditions
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
DERMA PELLA ACNE CARE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-795 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 18 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) LAURETH-7 (UNII: Z95S6G8201) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TEA TREE OIL (UNII: VIF565UC2G) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-795-01 1 in 1 CARTON 03/30/2023 1 25 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/30/2023 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)