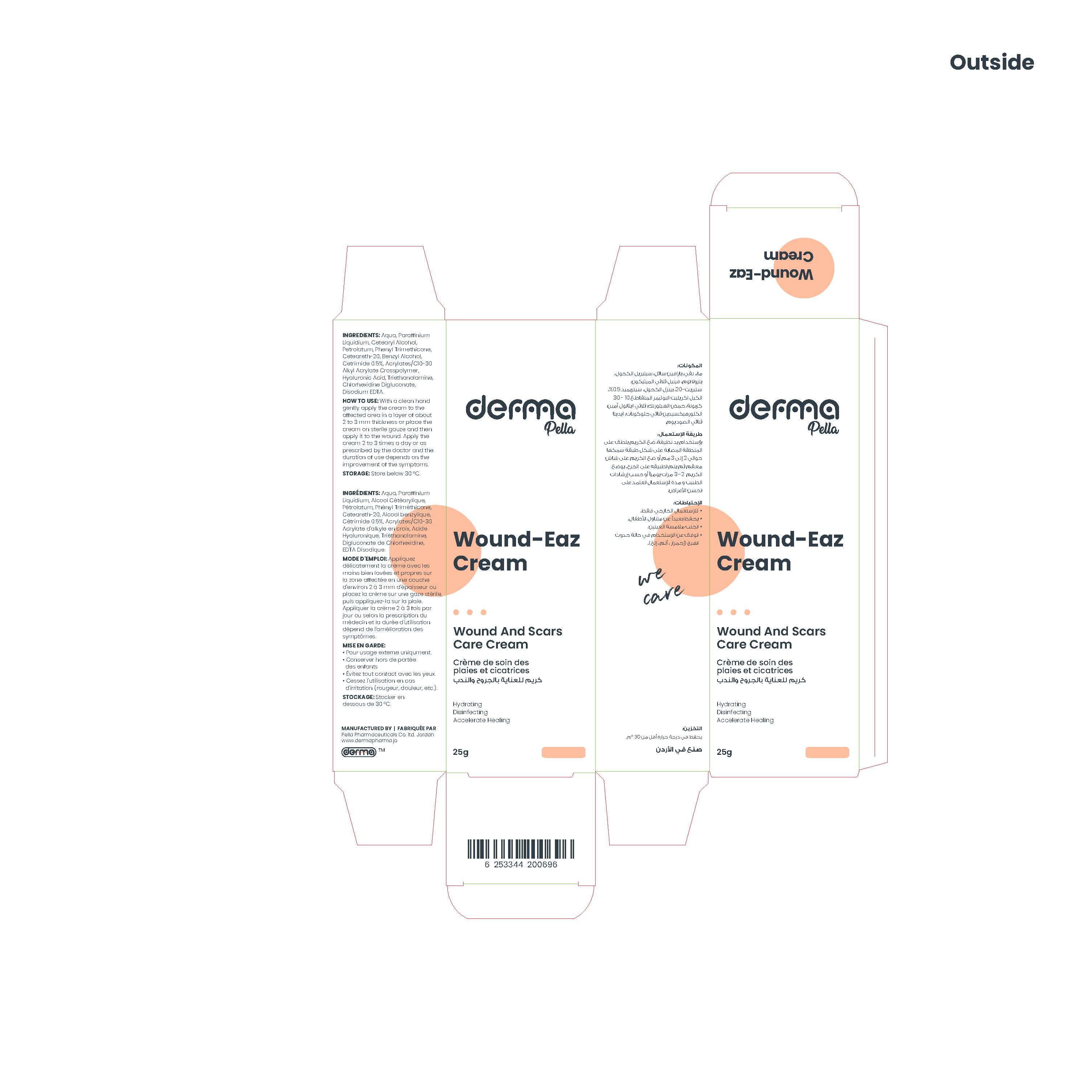
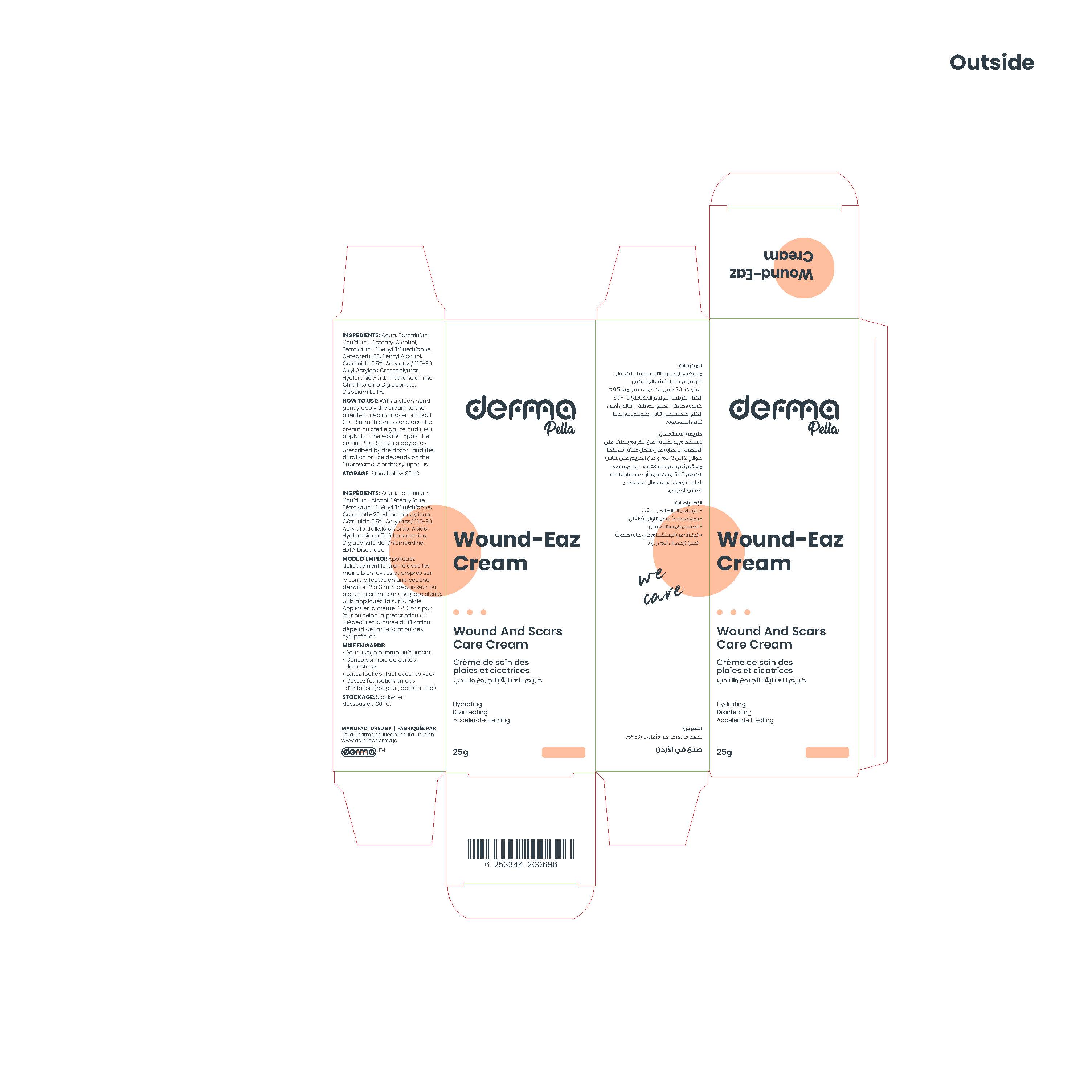
Label: DERMA WOUND-EAZ- cetrimide cream
- NDC Code(s): 82160-696-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Form and Presentation
- Active Ingredient
- Inactive Ingredients
- Purpose
-
Properties
This product is for the management of wounds and scars, it provides deep skin hydration, accelerates the process of regeneration. It works by softening and flattening raised scars of any kind and is an effective antiseptic with a wide range of activity against micro-organisms, including gram-positive and gram-negative bacteria, fungi, and viruses.
- Indications
- Precaution
-
Warnings
- For external use only.
- Avoid contact with eyes, if accidentally splashed into the eye, the open eye should be irrigated for at least 10 minutes.
- Discontinue use if irritation (redness, pain, etc.) occurs. If skin irritation occurs, consult your physician if the symptoms persist for more than 48 hr. - Contraindications
- Side Effects
-
Pregnancy and Lactation
There are no adequate data from the use of chlorhexidine digluconate and cetrimide in pregnant women.
The potential risk for humans is unknown but is most likely very low since chlorhexidine digluconate and cetrimide are poorly absorbed following topical application.
It is not known whether chlorhexidine digluconate and cetrimide are excreted in breast milk. There are no adequate data from the use of chlorhexidine and cetrimide in breastfeeding women. However, it is unlikely that the products are excreted in breast milk since the products are poorly absorbed. After topical usage of the product, as a general precaution, rinse nipples thoroughly with water before breastfeeding.
-
Dosage and Administration
With a clean hand gently apply the cream to the affected area in a layer of about 2 to 3 mm thickness or place the cream on sterile gauze and then apply it to the wound.
Apply the cream 2 to 3 times a day or as prescribed by the doctor and the duration of use depends on the improvement of the symptoms.
- Interactions
- Storage Conditions
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
DERMA WOUND-EAZ
cetrimide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-696 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETRIMIDE (UNII: 24QSH2NL8N) (CETRIMIDE - UNII:24QSH2NL8N) CETRIMIDE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) TROLAMINE (UNII: 9O3K93S3TK) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PETROLATUM (UNII: 4T6H12BN9U) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-696-01 1 in 1 CARTON 10/31/2022 1 25 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/31/2022 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)