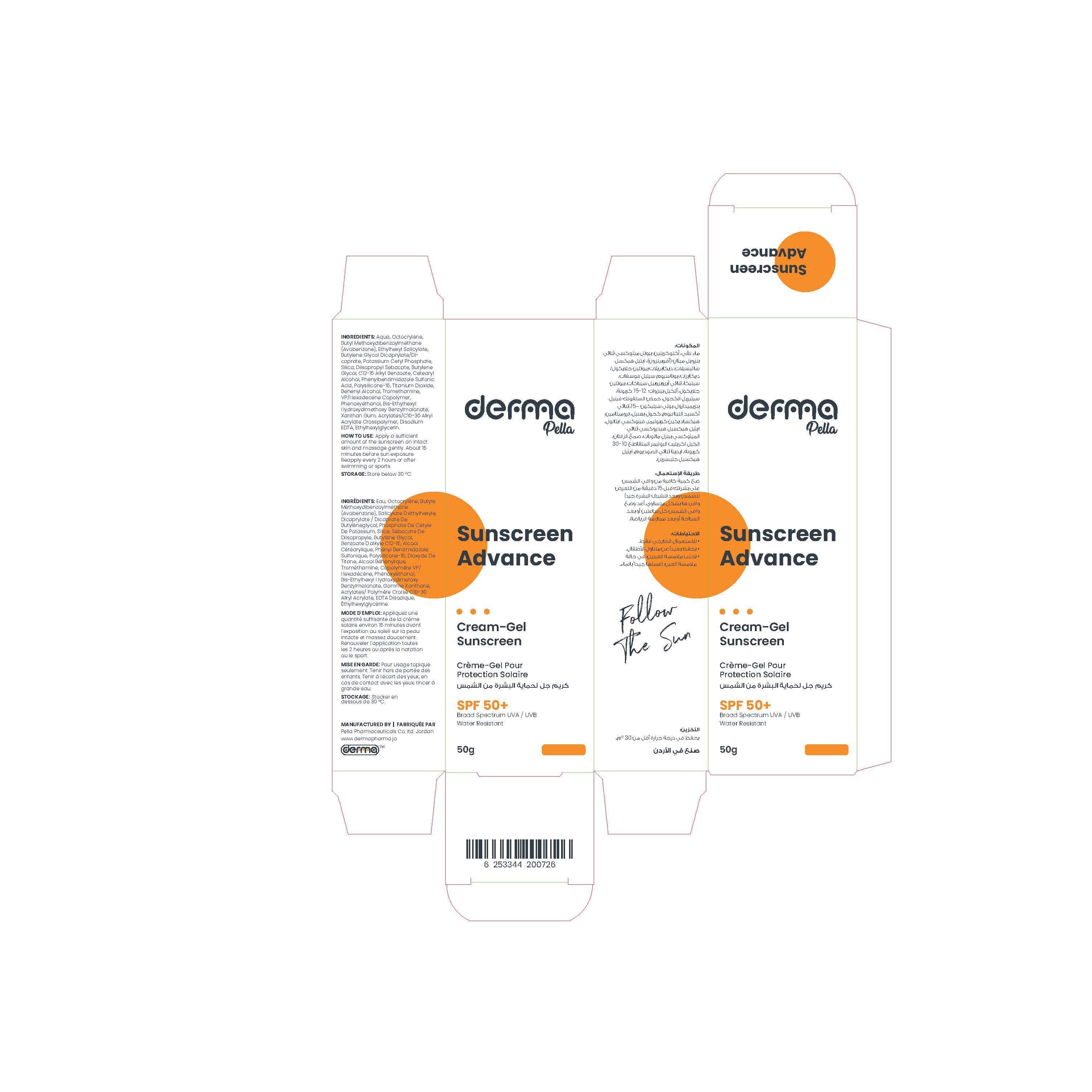
Label: DERMA PELLA SUNSCREEN ADVANCE CREAM-GEL- octocrylene, avobenzone, ethylhexyl salicylate, phenylbenzimidazole sulfonic acid, polysilicone-15, titanium dioxide cream
- NDC Code(s): 82160-726-01
- Packager: Pella Pharmaceuticals Co. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Form and Presentation
- Active Ingredients
-
Inactive Ingredients
Aqua, Butylene Glycol Dicaprylate/Dicaprate, Potassium Cetyl Phosphate, Silica, Diisopropyl Sebacate, Butylene Glycol, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Behenyl Alcohol, Tromethamine, VP/Hexadecene Copolymer, Phenoxyethanol, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Xanthan Gum, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Disodium EDTA, Ethylhexylglycerin
- Purpose
-
Properties
This product is developed to be suitable for all types of skin, with high sun protection factors (SPF 50 +) and optimum combination of UVB/UVA filters, they efficiently protect the skin pigmentation spots and skin ageing.
It is fast absorbing with dry touch and matte feel for optimal protection without leaving any white residue on skin.
Paraben Free, Water resistant.
- Indications
- Precautions
- Warnings
- Contraindications
- Side Effects
- Dosage and Administration
- Storage Conditions
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
DERMA PELLA SUNSCREEN ADVANCE CREAM-GEL
octocrylene, avobenzone, ethylhexyl salicylate, phenylbenzimidazole sulfonic acid, polysilicone-15, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82160-726 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 20 mg in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 16 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g POLYSILICONE-15 (UNII: F8DRP5BB29) (POLYSILICONE-15 - UNII:F8DRP5BB29) POLYSILICONE-15 20 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) DOCOSANOL (UNII: 9G1OE216XY) HEXADECYL POVIDONE (UNII: AG75W62QYU) PHENOXYETHANOL (UNII: HIE492ZZ3T) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) XANTHAN GUM (UNII: TTV12P4NEE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TROMETHAMINE (UNII: 023C2WHX2V) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82160-726-01 1 in 1 CARTON 12/26/2022 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 12/26/2022 Labeler - Pella Pharmaceuticals Co. Ltd (562370925)