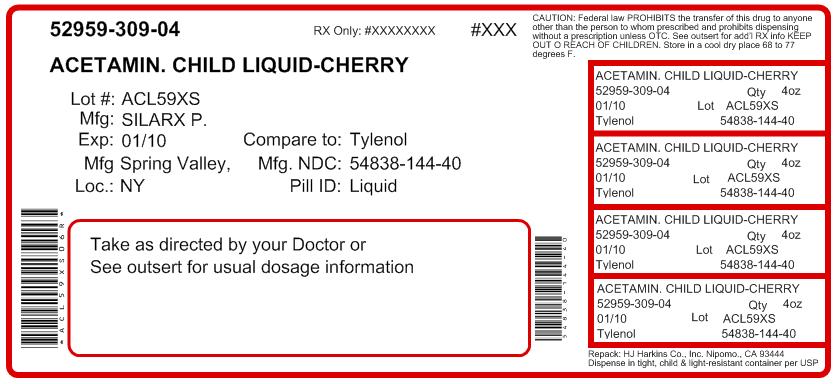
Label: ACETAMINOPHEN liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-309-04 - Packager: H.J. Harkins Company, Inc.
- This is a repackaged label.
- Source NDC Code(s): 54838-144
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 8, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
Warnings
Liver Warning:This product contains acetaminophen. Severe liver damage may occur if you take:
-
more than 5 of the recommended doses per day
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Alcohol warning:If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take acetaminophen or other pain relievers/fever reducers.
-
more than 5 of the recommended doses per day
-
Directions
- do not take more than directed (see overdose warning).
- if needed, repeat dose every 4 hours or as directed by a doctor
- do not give more than 5 doses in 24 hours
Other informationchildren under 2 yrs (under 24 lbs)
ask a doctor
children 2-3 years (24-35 lbs)
1 teaspoonful (5 mL)
children 4-5 years (36-47 lbs)
1 1/2 teaspoonfuls (7.5 mL)
children 6-8 years (48-59 lbs)
2 teaspoonfuls (10 mL)
children 9-10 years (60-71 lbs)
2 1/2 teaspoonfuls (12.5 mL)
children 11 years (72-95 lbs)
3 teaspoonfuls (15 mL)
adults & children 12 years & older
4 teaspoonfuls (20 mL)
Store at room temperature 20°-25°C (68°-77°F)
- do not take more than directed (see overdose warning).
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52959-309(NDC:54838-144) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) D&C red no. 33 (UNII: 9DBA0SBB0L) FD&C red no. 40 (UNII: WZB9127XOA) methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) saccharin sodium (UNII: SB8ZUX40TY) sodium benzoate (UNII: OJ245FE5EU) water (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor CHERRY (cherry flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-309-04 118 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/05/1994 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations Silarx Pharmaceuticals, Inc 161630033 manufacture