Label: ORALTAG- iohexol for solution
-
NDC Code(s):
54702-501-21,
54702-501-51,
54702-501-52,
54702-501-62, view more54702-501-98, 54702-501-99
- Packager: Interpharma Praha, a.s.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORALTAG safely and effectively. See full prescribing information for ORALTAG.
ORALTAG™ (iohexol) for oral solution
Initial U.S. Approval: 1985INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
For oral solution: Each single use bottle contains 9.7 g iohexol (equivalent to 4.5 g carbon bound iodine) (3)
CONTRAINDICATIONS
Hypersensitivity to iodinated contrast agents, including iohexol (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (incidence < 2%) are nausea, vomiting, and diarrhea ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Interpharma Praha at 1-877-886-7040 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Parenteral Administration
5.2 Hypersensitivity Reactions
5.3 Alteration of Thyroid Function Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
For oral use only[see Warnings and Precautions (5.1)]
Refer to Table 1 for dosing information.
Table 1 Dosing Guidelines for Oraltag
- *
- Total volume of Oraltag administered will vary depending on the size of the patient
Patient Age
Recommended Dose *
Volume of Prepared
Solution to Administer
(at a concentration of
9 mgI per mL)Maximum
Total Iodine
DoseAdults
Administer 4.5 g to 9 g of iodine (1 to 2 bottles of prepared solution), orally
500 mL to 1000 mL
9 grams
3 to 18 years of age
Administer up to 9 g of iodine (from less than 1 bottle up to 2 bottles of prepared solution), orally
280 mL to 750 mL,
depending on size of patient9 grams
Less than 3 years of age
Administer up to 4.5 g of iodine (portion of 1 bottle of prepared solution), orally
120 mL to 300 mL,
depending on size of patient4.5 grams
The variables of patient age, weight, or medical condition, may require adjustment of the concentration and/or volume of solution to be prepared for administration. If it is anticipated that the patient will have difficulty in consuming the required volume, a higher concentration of solution (up to 21 mgI per mL) can be prepared and a smaller volume administered (see Table 2).
Table 2 Preparation of Higher Concentrations of Oraltag at Lower Volumes
2.2 Preparation and Administration Instructions
- Reconstitute Oraltag, supplied as a powder in a single use bottle, with water or other beverages just before its use
- Do not mix other pharmaceuticals with Oraltag
- Use the 5 fill lines premolded and labeled on the bottle to determine the volume for the 5 target concentrations (9, 12, 15, 18, and 21 mgI/mL)
- Administer Oraltag 20 to 60 minutes before image acquisition
- Protect prepared solution from strong daylight and direct exposure to sunlight
- Discard any unused portions
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Oraltag is contraindicated in patients with a known hypersensitivity to iodinated contrast agents, including iohexol [see Warnings and Precautions (5.2)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Parenteral Administration
Oraltag is not a sterile product and is not suitable for a parenteral route of administration. Serious adverse reactions such as sepsis can occur if administered parenterally. Do not administer Oraltag parenterally.
5.2 Hypersensitivity Reactions
Administration of Oraltag can cause life-threatening hypersensitivity reactions including anaphylaxis [see Contraindications (4)] . Patients at increased risk include those with a previous reaction to an iodinated contrast agent and allergic disorders (i.e., bronchial asthma, allergic rhinitis, and food allergies). Emergency resuscitation equipment and trained personnel should be available.
-
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
In studies involving 44 adult and 69 pediatric patients who received oral and intravenous iohexol for CT examinations of the abdomen, two reports of vomiting (2%) were noted.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported following oral administration of the dilute, hypotonic solutions of iohexol (9 mgI/mL to 21 mgI/mL):
- Gastrointestinal: nausea, diarrhea
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no human data on risks associated with the use of Oraltag during pregnancy. The background risk in the U.S. general population of major birth defects is 2% to 4% and risk of miscarriage is 15% to 20% of clinically recognized pregnancies. In animal reproduction studies, no evidence of fetal harm was observed with intravenous administration of iohexol to rats and rabbits at doses up to 100 times the maximum recommended human intravenous dose.
8.2 Lactation
Risk Summary
Iohexol administered intravenously is present in human milk at concentrations approximately 0.5% of the maternal dose; however, it is not known to what extent iohexol administered orally is present in human milk. Iodinated contrast is poorly excreted into human milk and is poorly absorbed by the gastrointestinal tract of a breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Oraltag and any potential adverse effects on the breastfed infant from Oraltag.
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast media is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 10 hours (approximately 5 half-lives) after Oraltag administration in order to minimize potential drug exposure to a breastfed infant.
-
11 DESCRIPTION
Oraltag (iohexol) is a radiographic contrast agent for oral solution. Oraltag is provided as a nonsterile white to off-white powder with 9.7 g iohexol (equivalent to 4.5 g of carbon bound iodine) in a 20-ounce beverage bottle. Each bottle is individually sealed in a foil laminated pouch. Oraltag consists of 100 percent iohexol and contains no excipients.
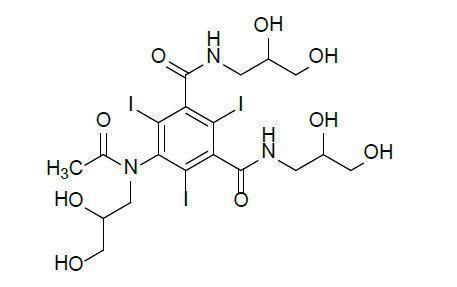
Iohexol is designated chemically as N,N´-bis(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodoisophthalamide. It is a nonionic, water-soluble iodinated contrast medium with a molecular weight of 821.14 (carbon bound iodine content 46.36%). In aqueous solution each triiodinated molecule remains undissociated.
The chemical structure is:
Oraltag, when prepared at a concentration of 9 mgI/mL in water, has an osmolality of 30 mOsmol/kg water. The calculated osmolality of 21 mgI/mL in water is 55 mOsmol/kg water. The prepared solutions are hypotonic.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Iohexol enhances imaging due to its high iodine content attenuating the beam of X-rays during CT examinations. Different tissues within the body attenuate X-rays to different degrees, and oral administration of iohexol allows for enhanced visualization due to the iodine present in bowel loops.
12.3 Pharmacokinetics
Orally administered iohexol is very poorly absorbed from the normal gastrointestinal tract. Only 0.1 to 0.5% of the oral dose is excreted by the kidneys. This amount may increase in the presence of bowel perforation, bowel obstruction, or severe inflammatory bowel disease.
Iohexol displays a low affinity for serum or plasma proteins and is poorly bound to serum albumin. No significant metabolism, deiodination or biotransformation occurs.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or mutagenesis.
In animal reproduction studies, no evidence of impaired fertility was observed with intravenous administration of iohexol to rats and rabbits at doses up to 100 times the maximum recommended human intravenous dose.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Oraltag is supplied as a nonsterile white to off-white powder for oral solution in a single-use 20-ounce polyethylene terephthalate beverage bottle closed with a lined polypropylene cap. Each bottle is packaged in a sealed foil pouch.
Twelve (12) bottles per pack (NDC 54702-501-52).
Twenty-five (25) bottles per pack (NDC 54702-501-62).
Storage
Store at controlled room temperature, 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect prepared solutions of Oraltag from strong daylight and direct exposure to sunlight.
Do not use if tamper-evident foil pouch has been opened.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients about the risk of hypersensitivity reactions to Oraltag and the need to notify their healthcare provider if signs or symptoms of hypersensitivity reaction occur [see Warnings and Precautions (5.2)] .
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 9.7 g Bottle Label
NDC 54702-501-21
Oraltag™
(iohexol) for oral solution
9.7 g of iohexol powder
(equivalent to 4.5 g of carbon bound iodine)
Single Use Bottle - Discard Unused PortionPrepared solution is 9-21 mgI/mL
For Oral Use Only
NonsterileFor indications and dosage, see prescribing information.
Store at 20-25˚C (68-77˚F); excursions permitted to 15-30˚C (59-86˚F) [see USP]
Protect prepared solutions of Oraltag from strong daylight and direct exposure to sunlight.
Rx Only
Manufactured for Interpharma Praha, a.s.
Prague, Czech Republic
by Ultra Seal Corporation
New Paltz, New York

-
PRINCIPAL DISPLAY PANEL - Foil Pouch Label
NDC 54702-501-51
Oraltag™
(iohexol) for oral solution
Contains a single-use bottle with 9.7 g of iohexol powder
(equivalent to 4.5 g of carbon bound iodine)For Oral Use Only
NonsterileStore at 20-25˚C (68-77˚F); excursions permitted to 15-30˚C (59-86˚F) [see USP]
Rx Only
Manufactured for Interpharma Praha, a.s.
Prague, Czech Republic
by Ultra Seal Corporation
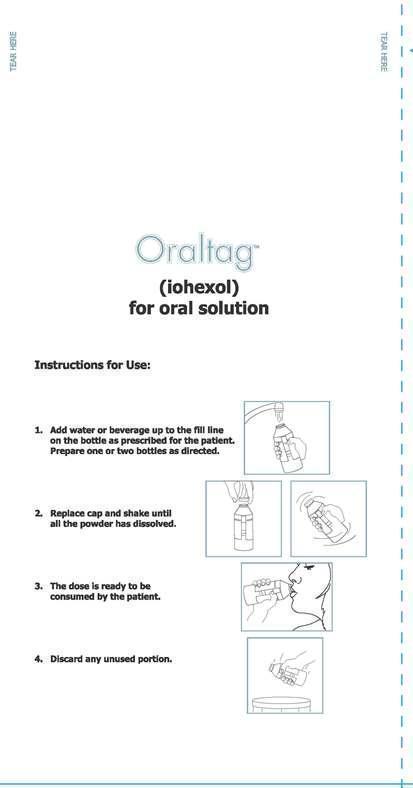
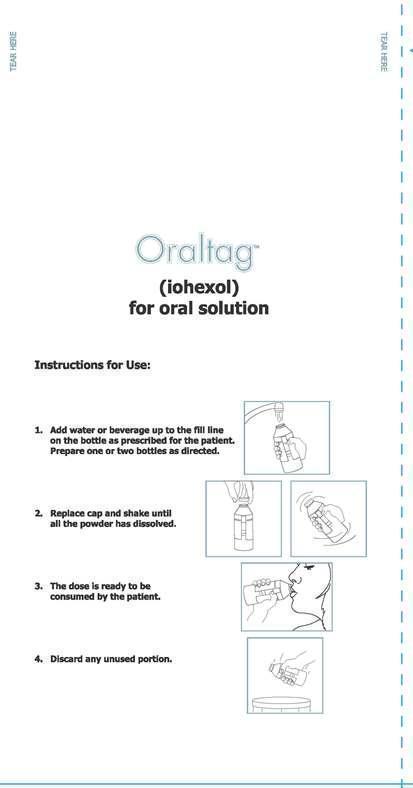
New Paltz, New YorkInstructions for Use:
1. Add water or beverage up to the fill line on the bottle as prescribed for the patient. Prepare one or two bottles as directed.
2. Replace cap and shake until all the powder has dissolved.
3. The dose is ready to be consumed by the patient.
4. Discard any unused portion.

-
INGREDIENTS AND APPEARANCE
ORALTAG
iohexol for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54702-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOHEXOL (UNII: 4419T9MX03) (IOHEXOL - UNII:4419T9MX03) IODINE 4.5 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54702-501-52 12 in 1 CASE 07/01/2016 1 NDC:54702-501-51 1 in 1 POUCH 1 NDC:54702-501-21 1 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:54702-501-62 25 in 1 CASE 07/01/2016 2 NDC:54702-501-51 1 in 1 POUCH 2 NDC:54702-501-21 1 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:54702-501-98 12 in 1 CASE 07/01/2016 3 NDC:54702-501-99 1 in 1 POUCH 3 NDC:54702-501-21 1 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205383 07/01/2016 Labeler - Interpharma Praha, a.s. (644354706) Establishment Name Address ID/FEI Business Operations Interpharma Praha, a.s. 644354706 api manufacture(54702-501) , analysis(54702-501) , manufacture(54702-501)