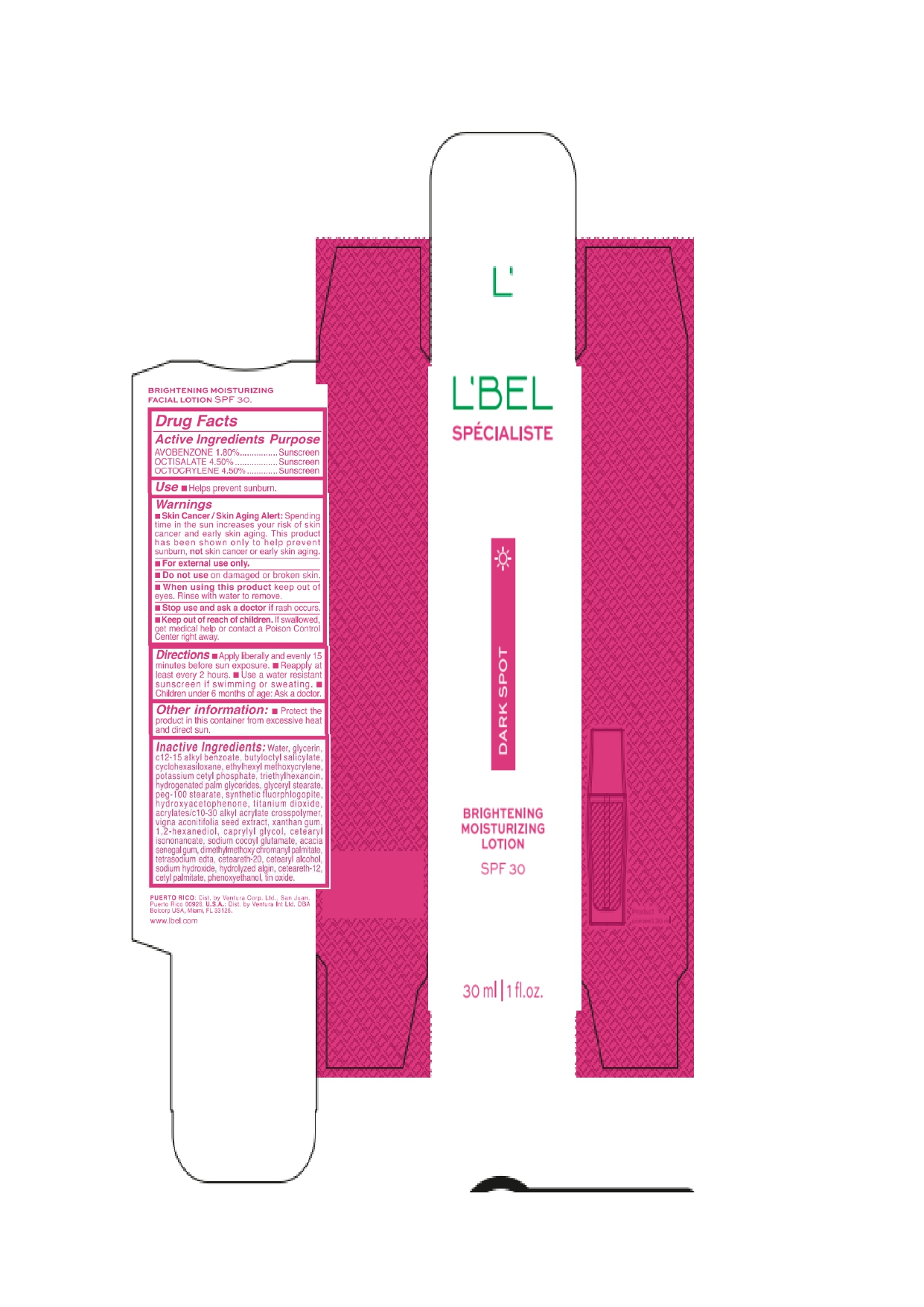
Label: LBEL SPECIALISTE DARK SPOT BRIGHTENING MOISTURIZING SPF 30- avobenzone, octisalate, octocrylene emulsion
- NDC Code(s): 14141-332-01
- Packager: BEL STAR SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, glycerin, c12-15 alkyl benzoate, butyloctyl salicylate, cyclohexasiloxane, ethylhexyl methoxycrylene, potassium cetyl phosphate, triethylhexanoin, hydrogenated palm glycerides, glyceryl stearate, peg-100 stearate, synthetic fluorphlogopite, hydroxyacetophenone, titanium dioxide, acrylates/c10-30 alkyl acrylate crosspolymer, vigna aconitifolia seed extract, xanthan gum, 1,2-hexanediol, caprylyl glycol, cetearyl isononanoate, sodium cocoyl glutamate, acacia senegal gum, dimethylmethoxy chromanyl palmitate, tetrasodium edta, ceteareth-20, cetearyl alcohol, sodium hydroxide, hydrolyzed algin, ceteareth-12, cetyl palmitate, phenoxyethanol, tin oxide.
- Company Information
- Package Label
-
INGREDIENTS AND APPEARANCE
LBEL SPECIALISTE DARK SPOT BRIGHTENING MOISTURIZING SPF 30
avobenzone, octisalate, octocrylene emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 18 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) MOTH BEAN (UNII: H7938ON8E5) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) STANNOUS OXIDE (UNII: JB2MV9I3LS) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) PHENOXYETHANOL (UNII: HIE492ZZ3T) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) CETYL PALMITATE (UNII: 5ZA2S6B08X) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE SODIUM (UNII: MP1J8420LU) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ACACIA (UNII: 5C5403N26O) CETEARYL ISONONANOATE (UNII: P5O01U99NI) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETEARETH-12 (UNII: 7V4MR24V5P) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-332-01 1 in 1 BOX 06/20/2023 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 06/20/2023 Labeler - BEL STAR SA (880160197)