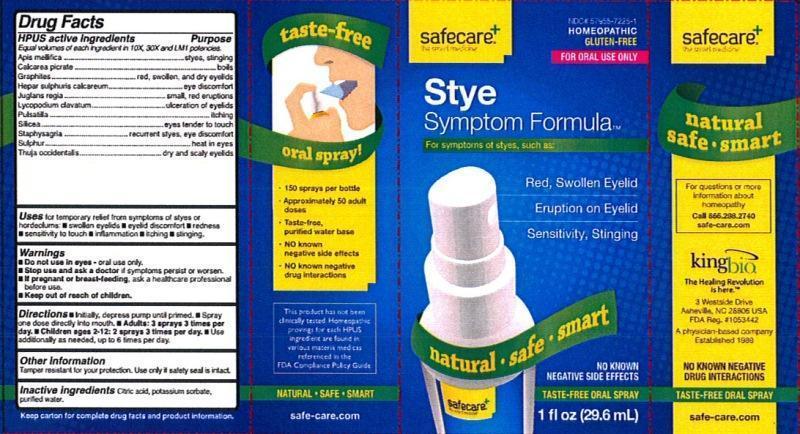
Label: STYE SYMPTOM FORMULA- apis mellifica, calcarea picrata, graphites, hepar sulphuris calcareum, juglans regia, lycopodium clavatum, pulsatilla, silicea, staphysagria, sulfur, thuja occidentalis liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-7225-1 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts
____________________________________________________________________________________________________________
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
HPUS active ingredients: Apis mellifica, Calcarea picrata, graphites, Hepar sulphuris calcareum, Juglans regia, Lycopodium clavatum, Pulsatilla, Silicea, Staphysagria, Sulphur, Thuja occidentalis.
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
PURPOSE
Drug Facts
__________________________________________________________________________________________________________________
HPUS active ingredients Purpose
Equal volumes of each ingredient in 10X, 30X, and LM1 potencies.
Apis mellifica.............................................................................styes, stinging
Calcarea picrata.........................................................................boils
Graphites..................................................................................red, swollen, and dry eyelids
Hepar sulphuris calcareum..........................................................eye discomfort
Juglans regia.............................................................................small, red eruptions
Lycopodium clavatum.................................................................ulceration of eyelids
Pulsatilla...................................................................................itching
Silicea......................................................................................eyes tender to touch
Staphysagria.............................................................................recurrent styes, eye discomfort
Sulphur....................................................................................heat in eyes
Thuja occidentalis......................................................................dry and scaly eyelids
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STYE SYMPTOM FORMULA
apis mellifica, calcarea picrata, graphites, hepar sulphuris calcareum, juglans regia, lycopodium clavatum, pulsatilla, silicea, staphysagria, sulfur, thuja occidentalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-7225 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 10 [hp_X] in 29.6 mL CALCIUM PICRATE (UNII: 53D441QVT8) (CALCIUM PICRATE - UNII:53D441QVT8) CALCIUM PICRATE 10 [hp_X] in 29.6 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] in 29.6 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 10 [hp_X] in 29.6 mL JUGLANS REGIA WHOLE (UNII: 87EJ76IO83) (JUGLANS REGIA WHOLE - UNII:87EJ76IO83) JUGLANS REGIA WHOLE 10 [hp_X] in 29.6 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 10 [hp_X] in 29.6 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 10 [hp_X] in 29.6 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 29.6 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 10 [hp_X] in 29.6 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 29.6 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 10 [hp_X] in 29.6 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-7225-1 1 in 1 CARTON 1 29.6 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/04/2013 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-7225)