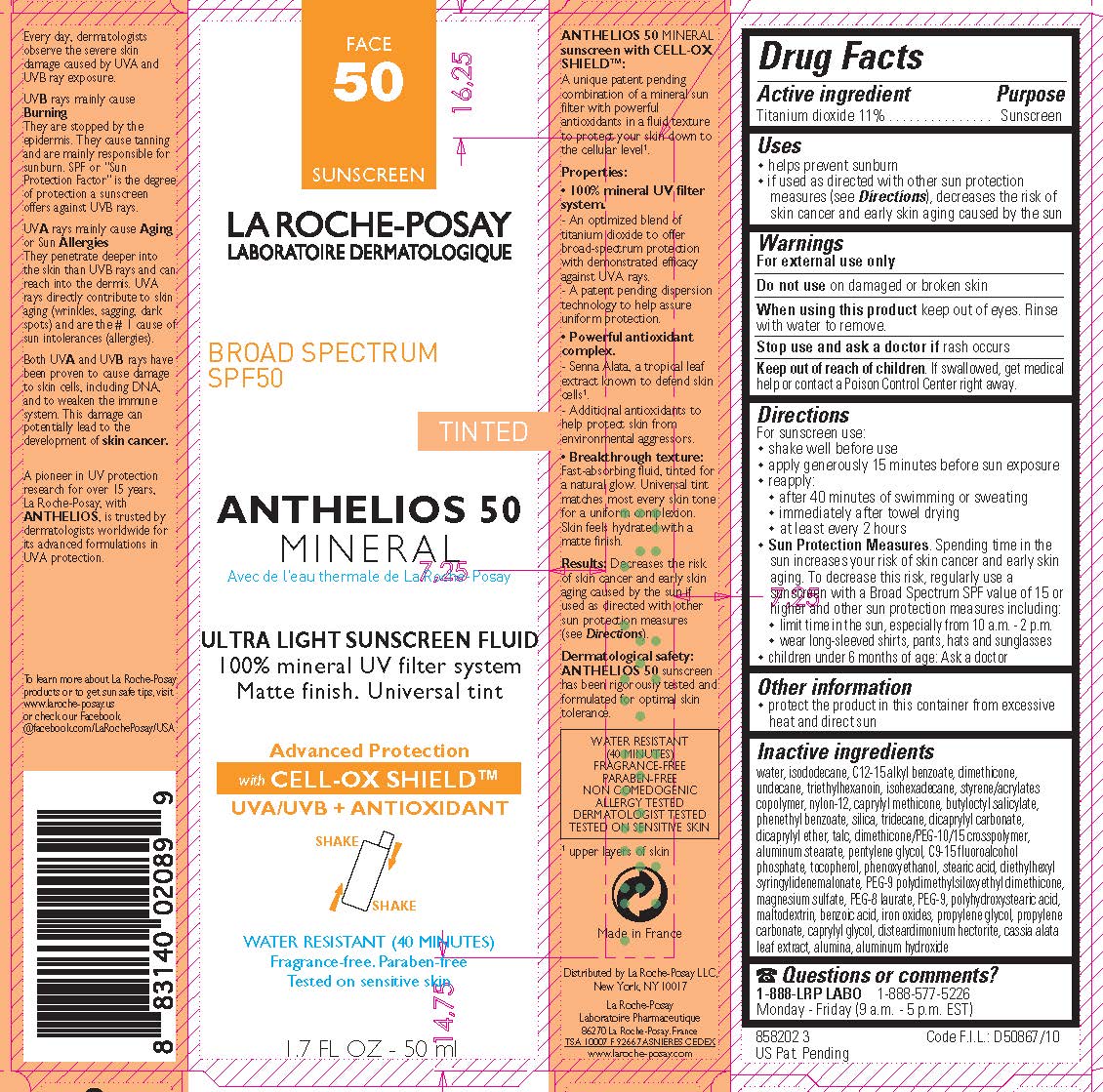
Label: LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 50 TINTED MINERAL SUNSCREEN BROAD SPECTRUM SPF 50- titanium dioxide lotion
- NDC Code(s): 69625-899-01, 69625-899-02
- Packager: Cosmetique Active Production
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
- shake well before use
- apply generously 15 minutes before sun exposure
- reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hoursSun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection meausures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, isododecane, C12-15 alkyl benzoate, dimethicone, undecane, triethylhexanoin, isohexadecane, styrene/acrylates copolymer, nylon-12, caprylyl methicone, butyloctyl salicylate, phenethyl benzoate, silica, tridecane, dicaprylyl carbonate, dicaprylyl ether, talc, dimethicone/PEG-10/15 crosspolymer, aluminum stearate, pentylene glycol, C9-15 fluoroalcohol phosphate, tocopherol, phenoxyethanol, stearic acid, diethylhexyl syringylidenemalonate, PEG-9 polydimethylsiloxyethyl dimethcone, magnesium sulfate, PEG-8 laurate, PEG-9, polyhydroxystearic acid, maltodextrin, benzoic acid, iron oxides, propylene glycol, propylene carbonate, caprylyl glycol, disteardimonim hectorite, cassia alata leaf extract, alumina, aluminum hydroxide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA ROCHE POSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 50 TINTED MINERAL SUNSCREEN BROAD SPECTRUM SPF 50
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69625-899 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 110 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69625-899-01 1 in 1 CARTON 06/01/2012 1 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69625-899-02 1 in 1 CARTON 06/01/2012 2 3 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2012 Labeler - Cosmetique Active Production (282658798) Establishment Name Address ID/FEI Business Operations Cosmetique Active Production 282658798 manufacture(69625-899) , pack(69625-899)