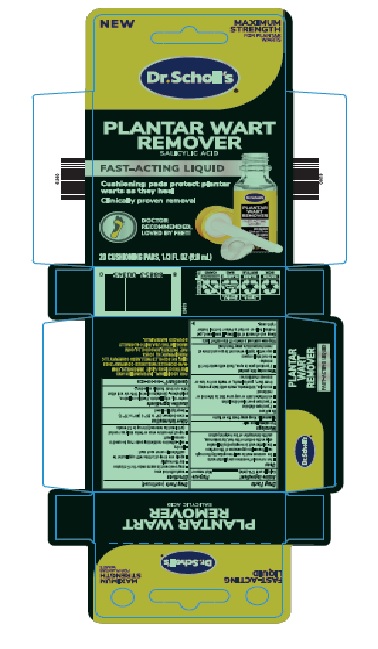
Label: WART REMOVER- salicylic acid liquid
- NDC Code(s): 73469-1192-0
- Packager: Scholls Wellness Company LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- wash affected area
- may soak wart in warm water for 5 minutes
- dry thoroughly
- apply one drop at a time with applicator to sufficiently cover each wart
- let dry
- sel-adhesive cushioning pads may be used to conceal wart
- repeat procedire once or twice daily as needed (until wart is removed) for up to 12 weeks
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WART REMOVER
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73469-1192 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1.7 mg in 10 mL Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) ETHYL LACTATE (UNII: F3P750VW8I) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) ETHER (UNII: 0F5N573A2Y) ALCOHOL (UNII: 3K9958V90M) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73469-1192-0 1 in 1 CARTON 05/31/2023 1 9.8 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 05/31/2023 Labeler - Scholls Wellness Company LLC (117174744) Registrant - Scapa Tapes North America LLC (079995435) Establishment Name Address ID/FEI Business Operations Scapa Tapes North America LLC 079995435 pack(73469-1192)