Label: MENTHOL, CAMPHOR gel
- NDC Code(s): 72937-006-40, 72937-006-80
- Packager: SUNSET NOVELTIES, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
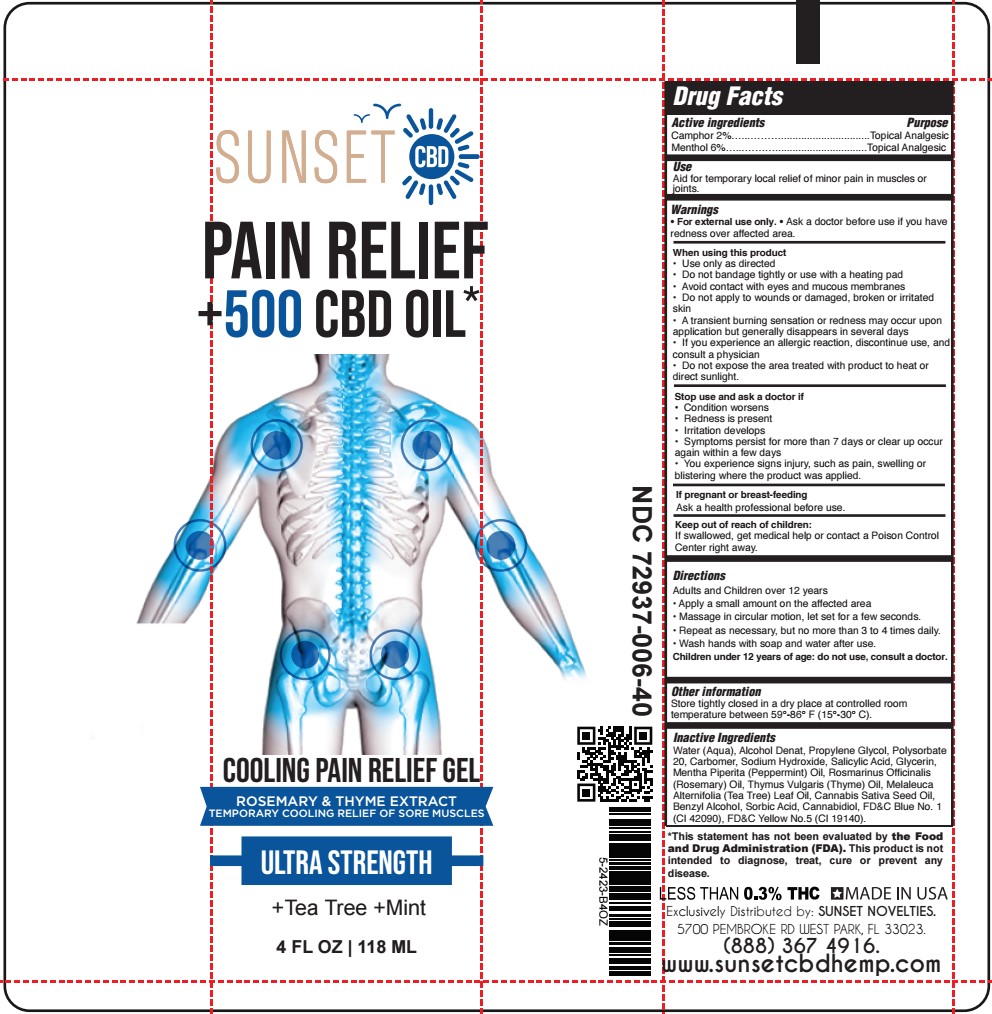
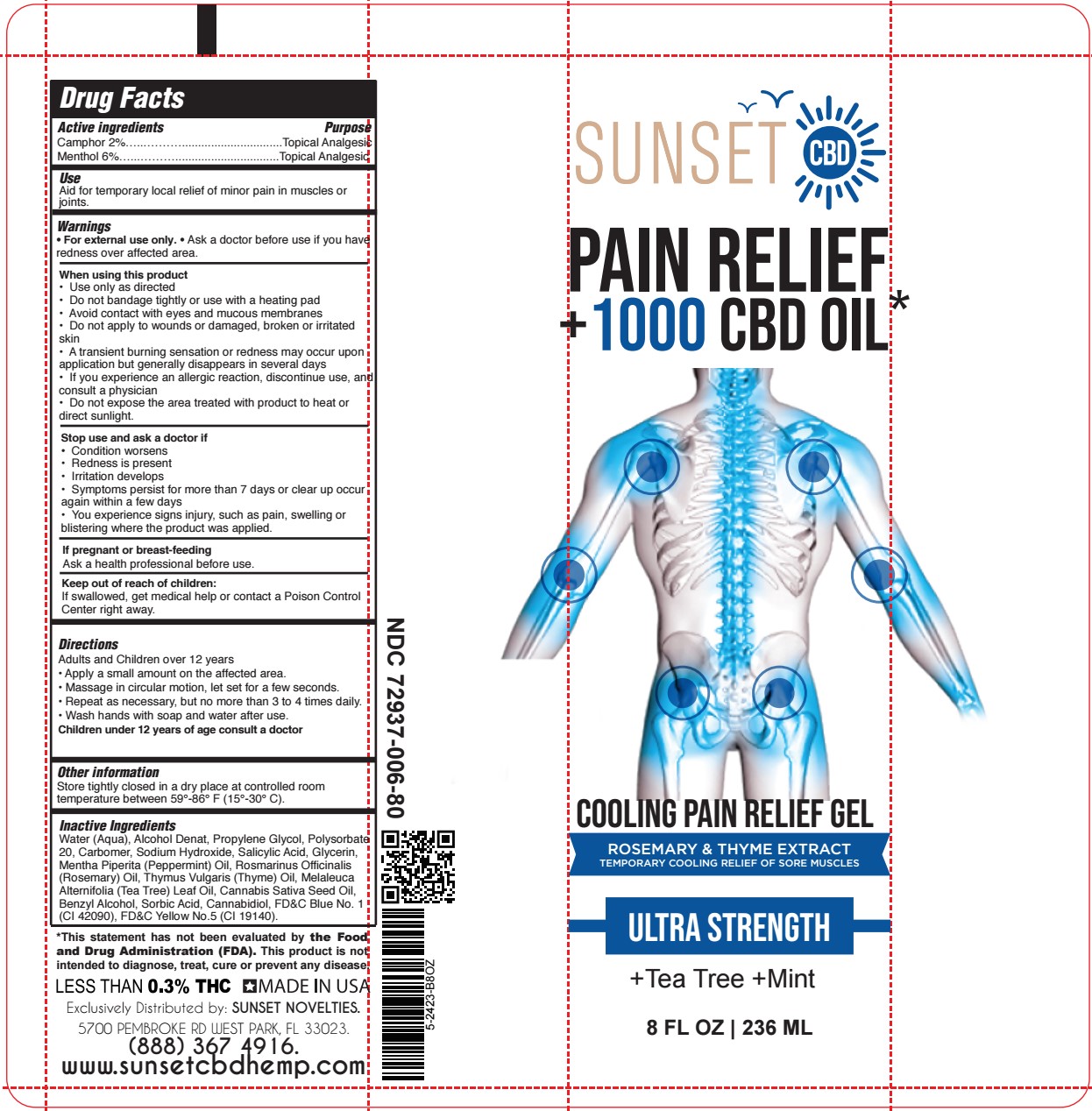
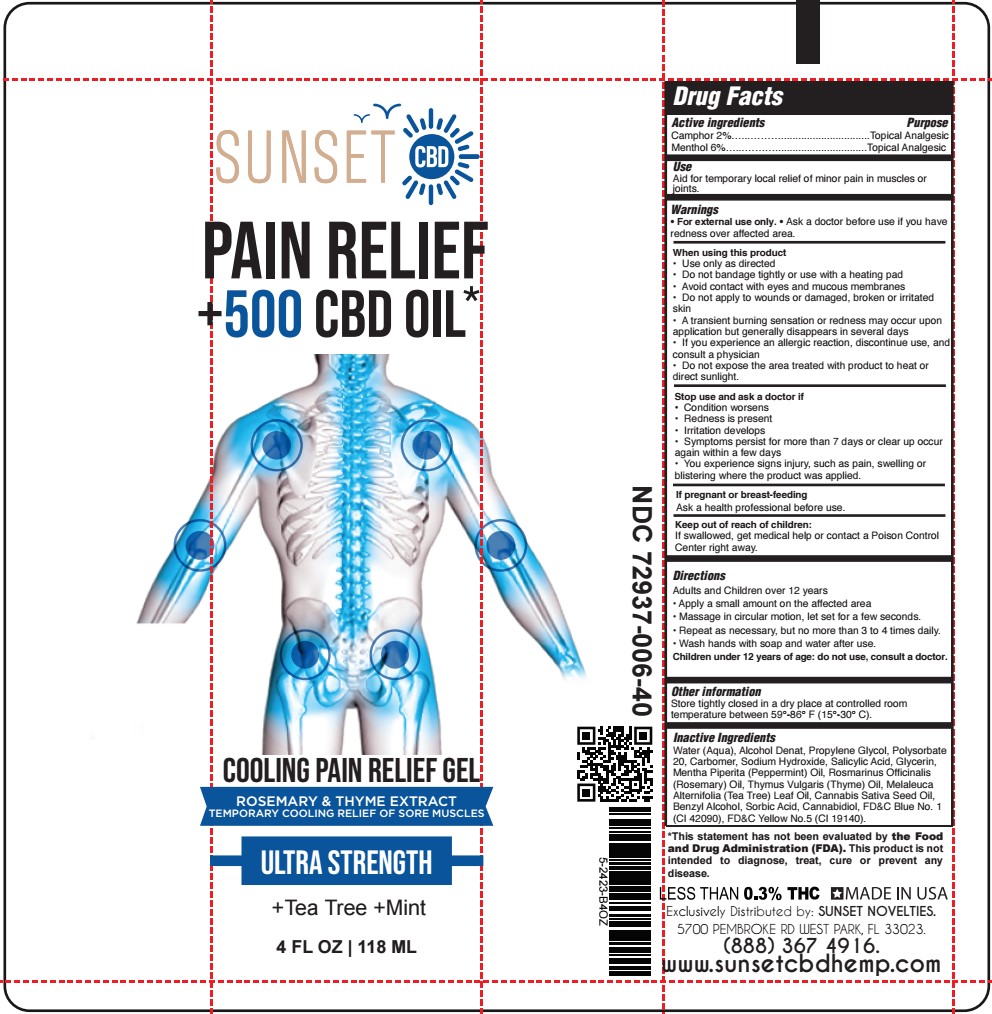
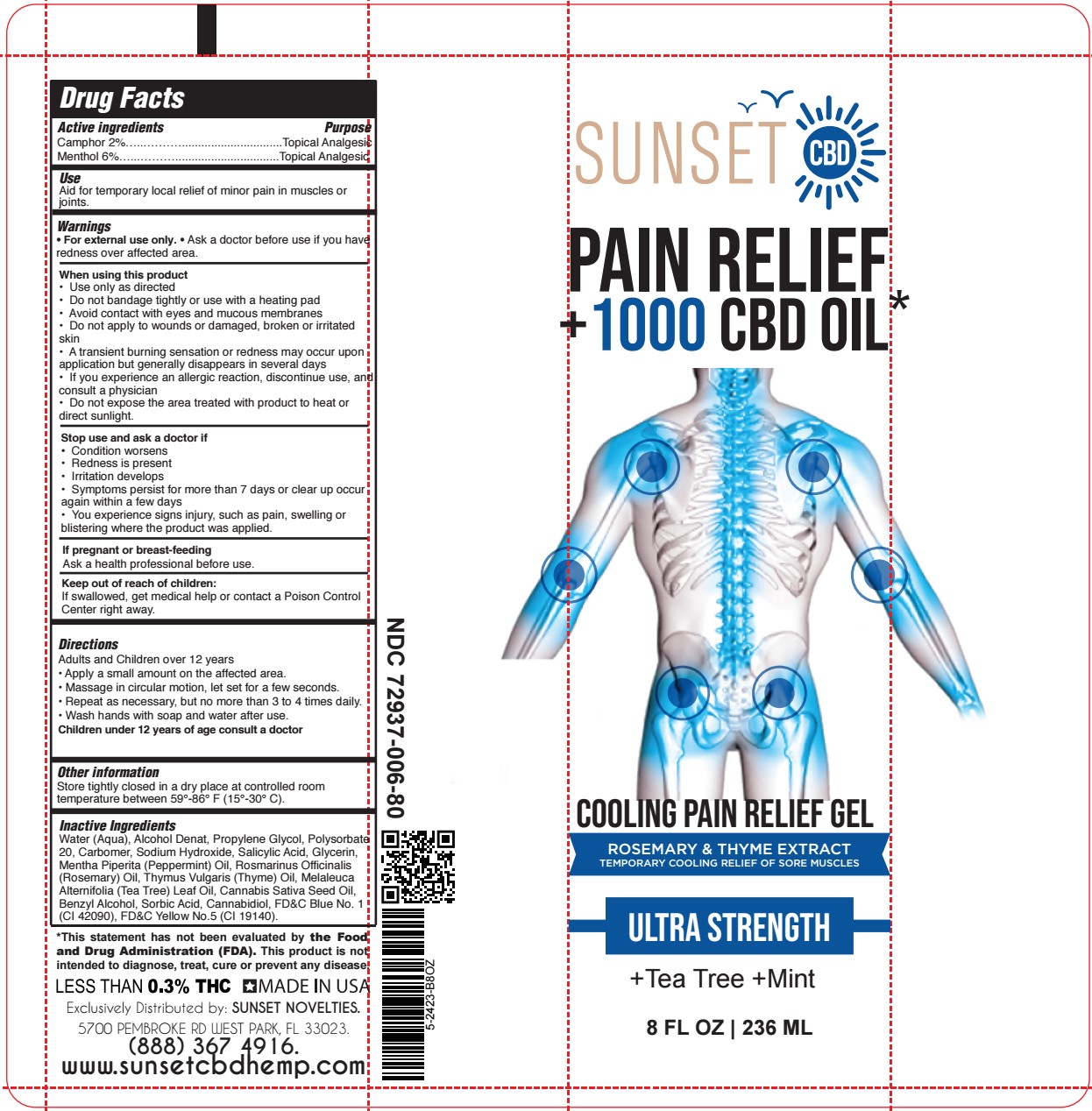
- ACTIVE INGREDIENT
- PURPOSE
- USES:
- WARNINGS
-
WHEN USING
Use only as directed
Do not bandage tightly or use with a heating pad
Avoid contact with eyes and mucous membranes
Do not apply to wounds or damaged, broken, or irritated skin
A transient burning sensation or redness may occur upon application but generally disappears in several days
If you experience an allergic reaction, discontinue use, and consult a physicianDo not expose the area treated with product to heat or direct sunlight.
- STOP USE AND ASK A DOCTOR IF:
- IF PREGNANT OR BREAST – FEEDING:
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS:
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Water (Aqua), Alcohol Denat, Propylene Glycol, Polysorbate 20, Carbomer, Sodium Hydroxide, Salicylic Acid, Glycerin, Mentha Piperita (Peppermint) Oil, Rosmarinus Officinalis (Rosemary) Oil, Thymus Vulgaris (Thyme) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Cannabis Sativa Seed Oil, Benzyl Alcohol, Sorbic Acid, Cannabidiol, FD&C Blue No. 1 (CI 42090), FD&C Yellow No.5 (CI 19140).
- SUNSET - COOLING PAIN RELIEF GEL 4oz TUBE
- SUNSET - COOLING PAIN RELIEF GEL 8oz TUBE
-
INGREDIENTS AND APPEARANCE
MENTHOL, CAMPHOR
menthol, camphor gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72937-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 100 g CAMPHOR, (-)- (UNII: 213N3S8275) (CAMPHOR, (-)- - UNII:213N3S8275) CAMPHOR, (-)- 2 g in 100 g Inactive Ingredients Ingredient Name Strength PEPPERMINT OIL (UNII: AV092KU4JH) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) CARBOMER 940 (UNII: 4Q93RCW27E) GLYCERIN (UNII: PDC6A3C0OX) SALICYLIC ACID (UNII: O414PZ4LPZ) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYSORBATE 20 (UNII: 7T1F30V5YH) ROSEMARY OIL (UNII: 8LGU7VM393) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBIC ACID (UNII: X045WJ989B) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM HYDROXIDE (UNII: 55X04QC32I) THYME OIL (UNII: 2UK410MY6B) MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) ALCOHOL (UNII: 3K9958V90M) CANNABIDIOL (UNII: 19GBJ60SN5) Product Characteristics Color white (TURQUOISE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72937-006-40 118 g in 1 TUBE; Type 0: Not a Combination Product 06/23/2023 2 NDC:72937-006-80 235 g in 1 TUBE; Type 0: Not a Combination Product 06/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/22/2020 Labeler - SUNSET NOVELTIES, INC (067218145)