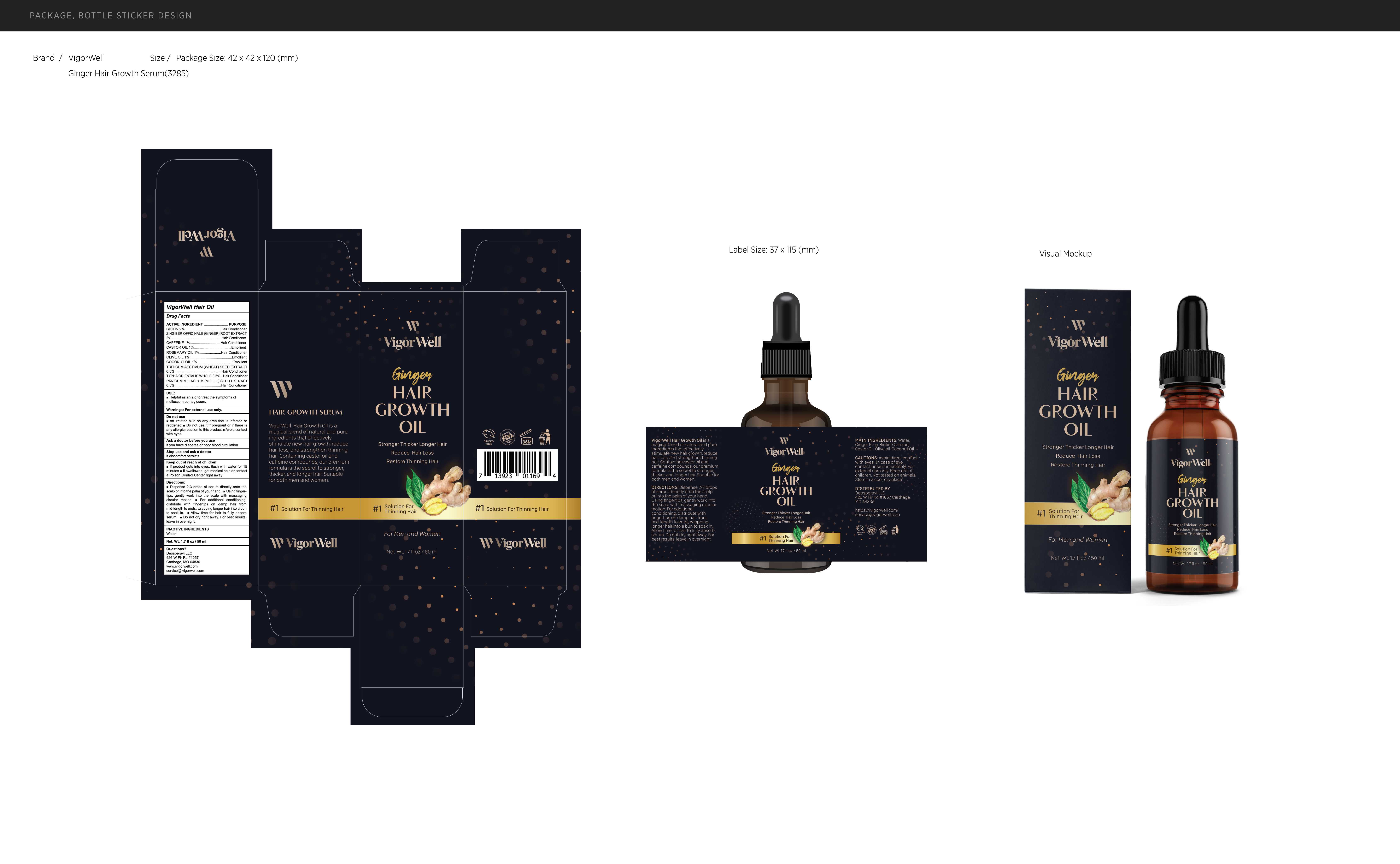
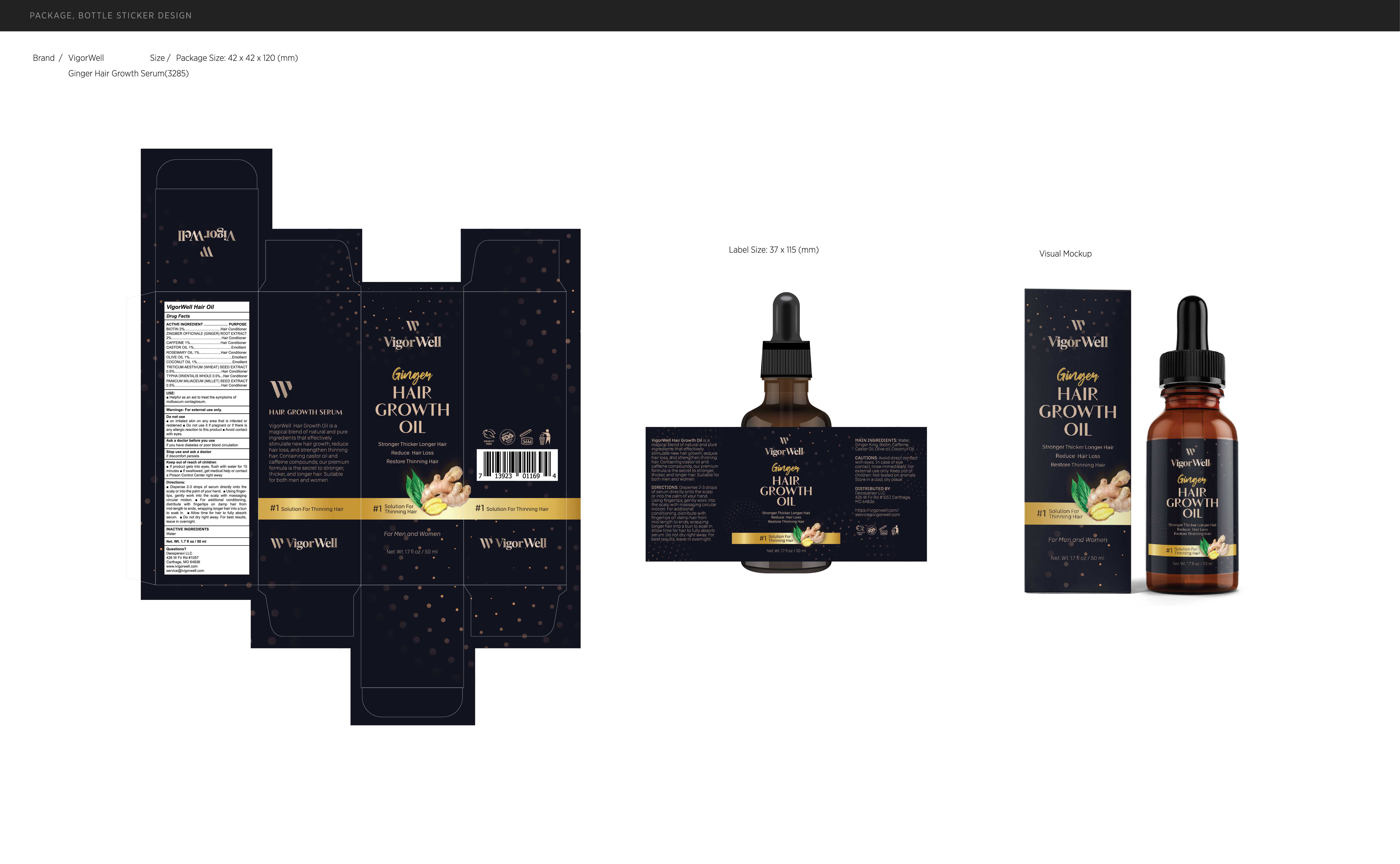
Label: VIGORWELL HAIR OIL- hair oil oil
- NDC Code(s): 82739-010-01
- Packager: Shenzhen Situya Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Stop Use
- Keep Oot Of Reach Of Children
-
Directions
Dispense 2-3 drops of serum directly onto thescalp or into the palm of your hand. Using finger-tips, gently work into the scalp with massagingcircular motion.For additional conditioning,distribute with fingertips on damp hair frommid-length to ends, wrapping longer hair into a bunto soak in.Allow time for hair to fully absorbserum. Do not dry right away. For best resultsleave in overnight.
- Inactive ingredients
- Question
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIGORWELL HAIR OIL
hair oil oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82739-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 1 mg in 100 mL OLIVE OIL (UNII: 6UYK2W1W1E) (OLIVE OIL - UNII:6UYK2W1W1E) OLIVE OIL 1 mg in 100 mL WHEAT (UNII: 4J2I0SN84Y) (WHEAT - UNII:4J2I0SN84Y) WHEAT 0.5 mg in 100 mL GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 2 mg in 100 mL ROSEMARY OIL (UNII: 8LGU7VM393) (ROSEMARY OIL - UNII:8LGU7VM393) ROSEMARY OIL 1 mg in 100 mL CASTOR OIL (UNII: D5340Y2I9G) (CASTOR OIL - UNII:D5340Y2I9G) CASTOR OIL 1 mg in 100 mL BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 2 mg in 100 mL COCONUT OIL (UNII: Q9L0O73W7L) (COCONUT OIL - UNII:Q9L0O73W7L) COCONUT OIL 1 mg in 100 mL TYPHA ORIENTALIS WHOLE (UNII: KF7V8V64ES) (TYPHA ORIENTALIS WHOLE - UNII:KF7V8V64ES) TYPHA ORIENTALIS WHOLE 0.5 mg in 100 mL MILLET (UNII: TJR6B3R47P) (MILLET - UNII:TJR6B3R47P) MILLET 0.5 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82739-010-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 05/25/2023 Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) Establishment Name Address ID/FEI Business Operations Shenzhen Situya Trading Co., Ltd. 706154255 label(82739-010) , manufacture(82739-010)