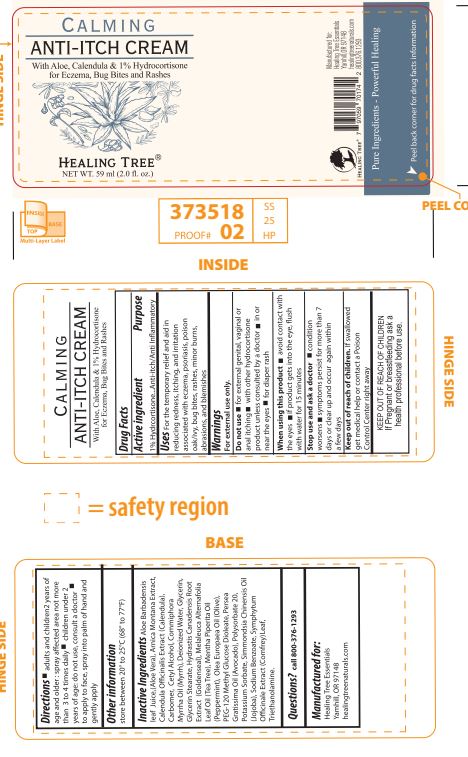
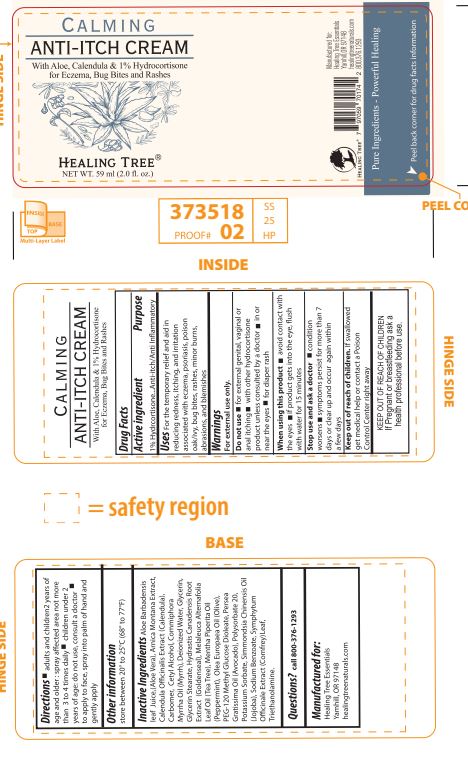
Label: HEALING TREE CALMING ANTI-ITCH- hydrocortisone cream
- NDC Code(s): 76348-804-05, 76348-804-06
- Packager: Renu Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- STATEMENT OF IDENTITY
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Aloe Barbadensis leaf juice (Aloe Vera), Arnica Montana Extract, Calendula Officinalis Extract (Calendula), Carbomer, Cetyl Alcohol,
Commiphora Myrrha Oil (Myrrh), Deionized Water, Glycerin, Glycerol Stearate,Hydrastis Canadensis Root Extract (Goldenseal),
Melaleuca Alternifolia Leaf Oil (Tea Tree), Mentha Piperita Oil (Peppermint), Olea Europaea Oil (Olive), PEG-120 Methyl Glucose
Dioleate, Persea Gratissima Oil (Avocado), Polysorbate 20, Potassium Sorbate, Simmondsia Chinensis Oil (Jojoba), Sodium
Benzoate, Symphytum Officinale Extract (Comfrey) Leaf, Triethanolamine.
- QUESTIONS
- Multi-layer Label
-
INGREDIENTS AND APPEARANCE
HEALING TREE CALMING ANTI-ITCH
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-804 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 0.59 mg in 59 mL Inactive Ingredients Ingredient Name Strength MYRRH OIL (UNII: H74221J5J4) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) PEPPERMINT OIL (UNII: AV092KU4JH) JOJOBA OIL (UNII: 724GKU717M) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) WATER (UNII: 059QF0KO0R) AVOCADO OIL (UNII: 6VNO72PFC1) OLIVE OIL (UNII: 6UYK2W1W1E) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 20 (UNII: 7T1F30V5YH) GOLDENSEAL (UNII: ZW3Z11D0JV) COMFREY (UNII: D05HXK6R3G) SODIUM BENZOATE (UNII: OJ245FE5EU) TROLAMINE (UNII: 9O3K93S3TK) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-804-06 1 in 1 BOX 05/26/2023 1 NDC:76348-804-05 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/26/2023 Labeler - Renu Laboratories, Inc. (945739449) Establishment Name Address ID/FEI Business Operations Renu Laboratories, Inc. 945739449 manufacture(76348-804)