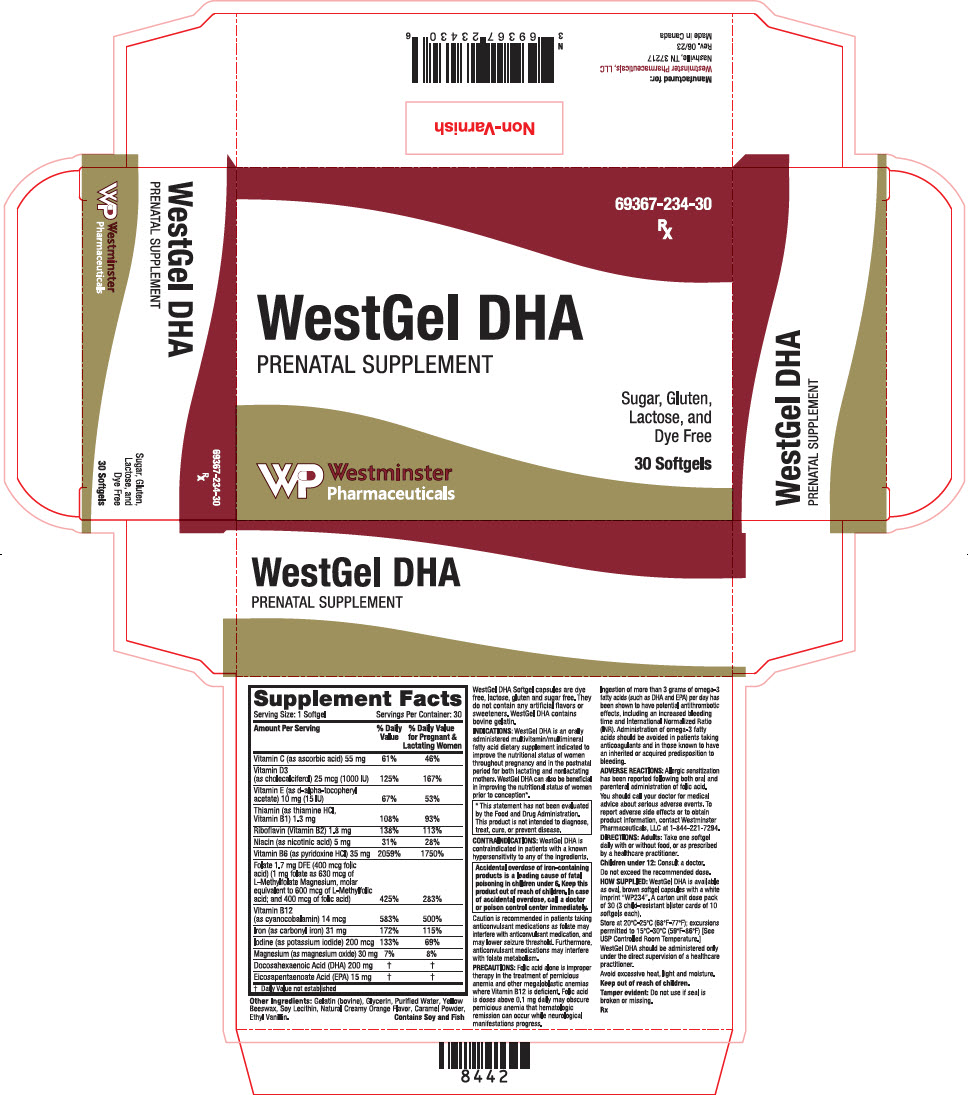
Label: WESTGEL DHA- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filled
- NHRIC Code(s): 69367-234-30
- Packager: Westminster Pharmaceuticals, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated July 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 Softgel Servings Per Container: 30 Amount Per Serving % Daily Value % Daily Value for Pregnant & Lactating Women - *
- Daily Value not established
Vitamin C (as ascorbic acid) 55 mg 61% 46% Vitamin D3
(as cholecalciferol) 25 mcg (1000 IU)125% 167% Vitamin E (as d-alpha-tocopheryl acetate) 10 mg (15 IU) 67% 53% Thiamin (as thiamine HCl, Vitamin B1) 1.3 mg 108% 93% Riboflavin (Vitamin B2) 1.8 mg 138% 113% Niacin (as nicotinic acid) 5 mg 31% 28% Vitamin B6 (as pyridoxine HCl) 35 mg 2059% 1750% Folate 1.7 mg DFE (400 mcg folic acid) (1 mg folate as 630 mcg of L-Methylfolate Magnesium, molar equivalent to 600 mcg of L-Methylfolic acid; and 400 mcg of folic acid) 425% 283% Vitamin B12
(as cyanocobalamin) 14 mcg583% 500% Iron (as carbonyl iron) 31 mg 172% 115% Iodine (as potassium iodide) 200 mcg 133% 69% Magnesium (as magnesium oxide) 30 mg 7% 8% Docosahexaenoic Acid (DHA) 200 mg * * Eicosapentaenoate Acid (EPA) 15 mg * * Other Ingredients: Gelatin (bovine), Glycerin, Purified Water, Yellow Beeswax, Soy Lecithin, Natural Creamy Orange Flavor, Caramel Powder, Ethyl Vanillin.
Contains Soy and Fish
WestGel DHA Softgel capsules are dye free, lactose, gluten and sugar free. They do not contain any artificial flavors or sweeteners. WestGel DHA contains bovine gelatin.
-
INDICATIONS
WestGel DHA is an orally administered multivitamin/multimineral fatty acid dietary supplement indicated to improve the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. WestGel DHA can also be beneficial in improving the nutritional status of women prior to conception1.
- 1
- This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent disease.
-
CONTRAINDICATIONS
WestGel DHA is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. Caution is recommended in patients taking anticonvulsant medications as folate may interfere with anticonvulsant medication, and may lower seizure threshold. Furthermore, anticonvulsant medications may interfere with folate metabolism.
-
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid is doses above 0.1 mg daily may obscure pernicious anemia that hematologic remission can occur while neurological manifestations progress.
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA and EPA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid. You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact Westminster Pharmaceuticals, LLC at 1-844-221-7294.
-
DIRECTIONS
HOW SUPPLIED
WestGel DHA is available as oval, brown softgel capsules with a white imprint "WP234". A carton unit dose pack of 30 (3 child-resistant blister cards of 10 softgels each).
Store at 20ºC-25ºC (68ºF-77ºF); excursions permitted to 15ºC-30ºC (59ºF-86ºF) [See USP Controlled Room Temperature.]
WestGel DHA should be administered only under the direct supervision of a healthcare practitioner.
Avoid excessive heat, light and moisture.
- PRINCIPAL DISPLAY PANEL - 30 Softgel Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
WESTGEL DHA
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacin, pyridoxine hydrochloride, levomefolate magnesium, folic acid, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, doconexent, and icosapent capsule, liquid filledProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69367-234 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 55 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 1000 [iU] .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 15 [iU] THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 1.3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.8 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 5 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 35 mg LEVOMEFOLATE MAGNESIUM (UNII: 1VZZ62R081) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 600 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 400 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 14 ug IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 31 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 200 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 30 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 200 mg ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 15 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) CARAMEL (UNII: T9D99G2B1R) ETHYL VANILLIN (UNII: YC9ST449YJ) YELLOW WAX (UNII: 2ZA36H0S2V) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ORANGE (UNII: 5EVU04N5QU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69367-234-30 3 in 1 CARTON 1 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 01/23/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 10 mm imprint flavor Labeler - Westminster Pharmaceuticals, LLC (079516651)