Label: NYSTATIN tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-1487-0 - Packager: Carilion Materials Management
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 15, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Nystatin is an antimycotic polyene antibiotic obtained from . Its structural formula: Streptomyces noursei
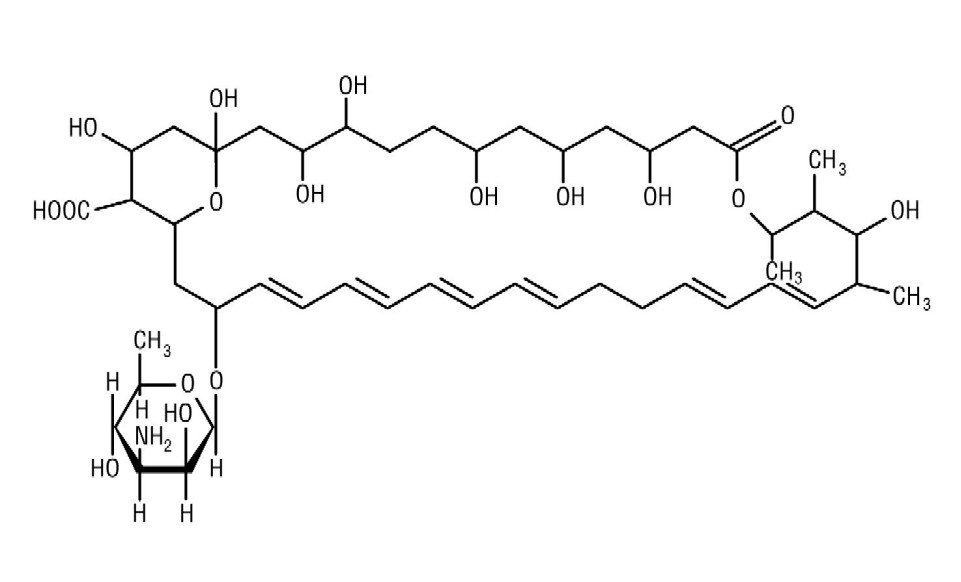
C H NO M.W. 926.13 477517
Nystatin tablets contain the inactive ingredients: Corn Starch, Povidone, Compressible Sugar, Microcrystalline Cellulose, Sodium Starch Glycolate, Talc, Magnesium Stearate, Purified Water, and Coloring.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology
Nystatin is both fungistatic and fungicidal against a wide variety of yeasts and yeast like fungi. demonstrates no significant resistance to nystatin on repeated subculture in increasing levels of nystatin; other species become quite resistant. Generally, resistance does not develop . Nystatin acts by binding to sterols in the cell membrane of susceptible species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses. in vitroCandida albicansin vitroCandidain vivoCandida
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
This medication is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. There also have been no studies to determine mutagenicity or whether this medication affects fertility in males or females.
-
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported (see ). PRECAUTIONS, General
Gastrointestinal
Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Nystatin 500,000 units
-
INGREDIENTS AND APPEARANCE
NYSTATIN
nystatin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68151-1487(NDC:0093-0983) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 500000 [USP'U] Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K29/32 (UNII: 390RMW2PEQ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) Product Characteristics Color BROWN Score no score Shape ROUND Size 10mm Flavor Imprint Code 93;983 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-1487-0 1 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062506 09/30/1990 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-1487)


