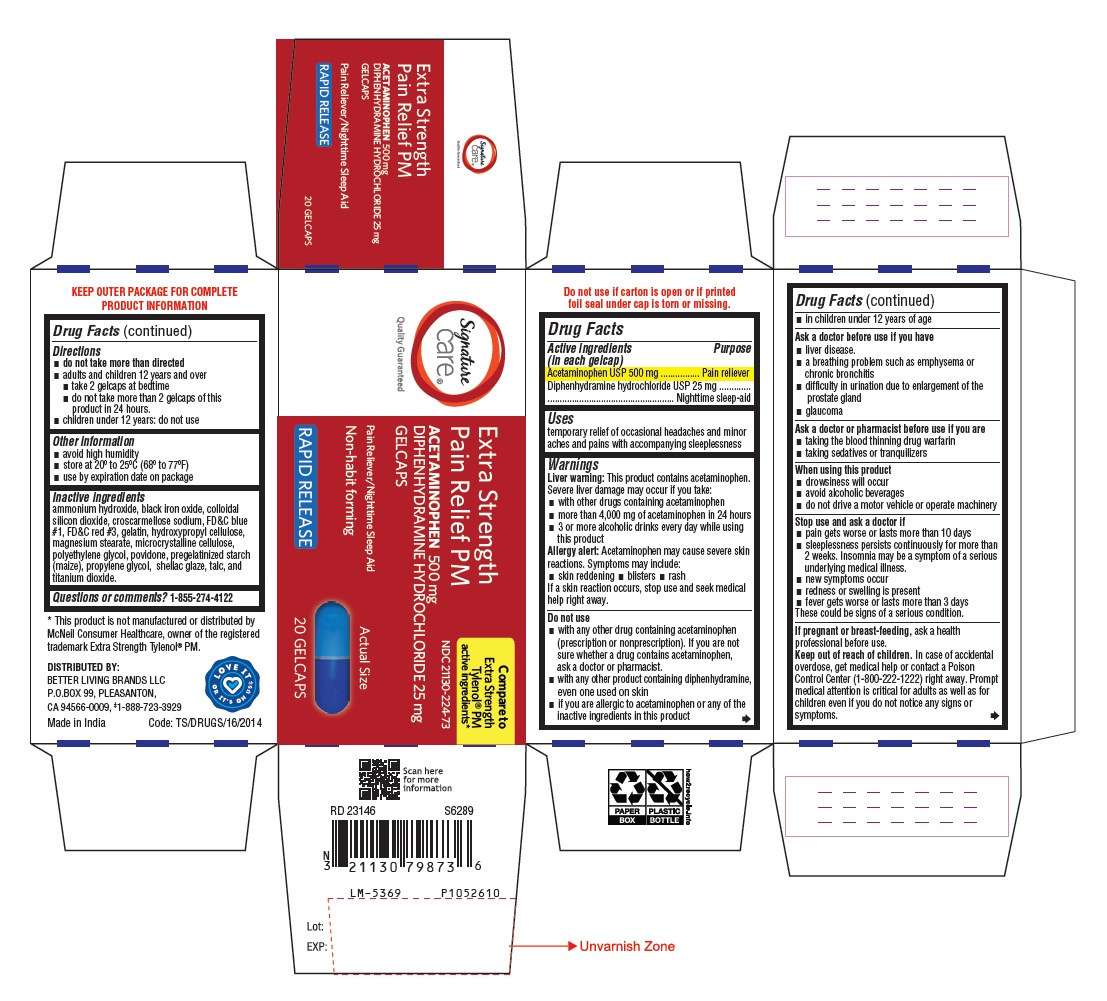
Label: ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE tablet
- NDC Code(s): 21130-224-18, 21130-224-73
- Packager: Better Living Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
-
Warnings
Liver warning:This product contains acetaminophen.
Severe liver damage may occur if you take:- with other drugs containing acetaminophen
- more than 4,000 mg of acetaminophen in 24 hours
- 3 or more alcoholic drinks every day while using this product
Allergy alert:Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
-
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- in children under 12 years of age
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
-
Inactive ingredients
ammonium hydroxide, black iron oxide, colloidal silicon dioxide, croscarmellose sodium, FD&C blue #1, FD&C red #3, gelatin, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch (maize), propylene glycol, shellac glaze, talc, and titanium dioxide.
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 500 mg / 25 mg (20 Caplets Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 500 mg / 25 mg (20 Caplets Bottle Carton)
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE
acetaminophen and diphenhydramine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-224 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) POVIDONE K90 (UNII: RDH86HJV5Z) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue (Dark blue and Light blue with white band) Score no score Shape CAPSULE (Biconvex) Size 20mm Flavor Imprint Code T;6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-224-73 1 in 1 CARTON 11/18/2023 1 20 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:21130-224-18 1 in 1 CARTON 11/18/2023 2 80 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 11/18/2023 Labeler - Better Living Brands, LLC (009137209) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650844777 analysis(21130-224) , manufacture(21130-224)