Label: CREST 3D WHITE BRILLIANCE LUMINOUS PURPLE- sodium fluoride paste, dentifrice
- NDC Code(s): 69423-787-35, 69423-787-46, 69423-787-85
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warning
-
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
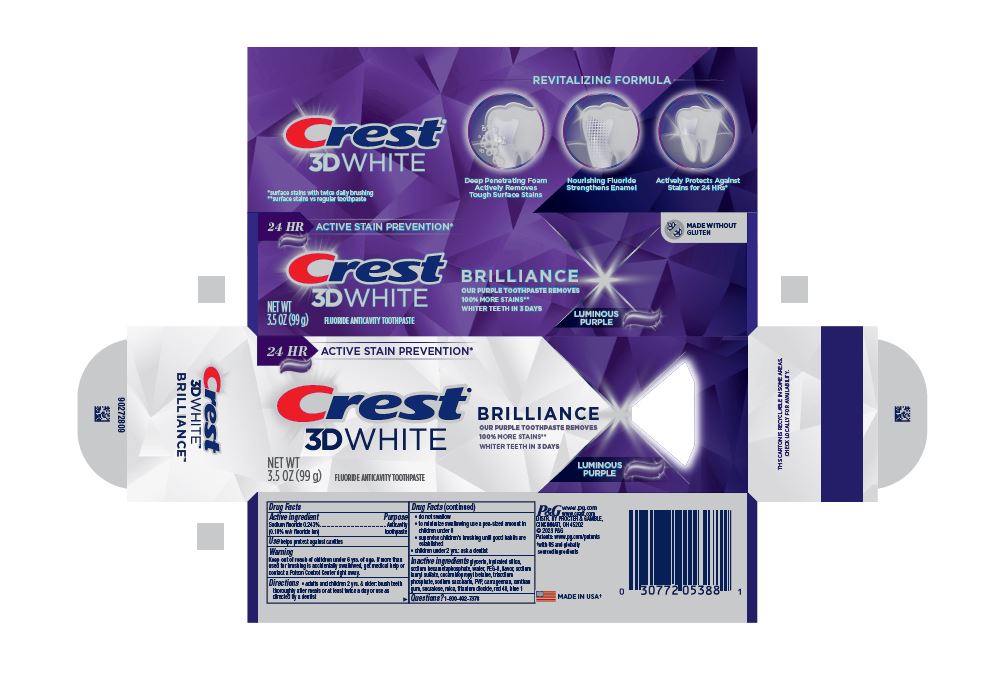
- PRINCIPAL DISPLAY PANEL - 99 g Tube Carton
-
INGREDIENTS AND APPEARANCE
CREST 3D WHITE BRILLIANCE LUMINOUS PURPLE
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-787 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.6 mg in 1 g Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) CARRAGEENAN (UNII: 5C69YCD2YJ) SUCRALOSE (UNII: 96K6UQ3ZD4) HYDRATED SILICA (UNII: Y6O7T4G8P9) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM PHOSPHATE, TRIBASIC, ANHYDROUS (UNII: SX01TZO3QZ) Product Characteristics Color purple Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-787-85 1 in 1 CARTON 06/15/2022 1 24 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69423-787-46 1 in 1 CARTON 06/15/2022 2 131 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:69423-787-35 1 in 1 CARTON 06/15/2022 3 99 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/15/2022 Labeler - The Procter & Gamble Manufacturing Company (004238200)

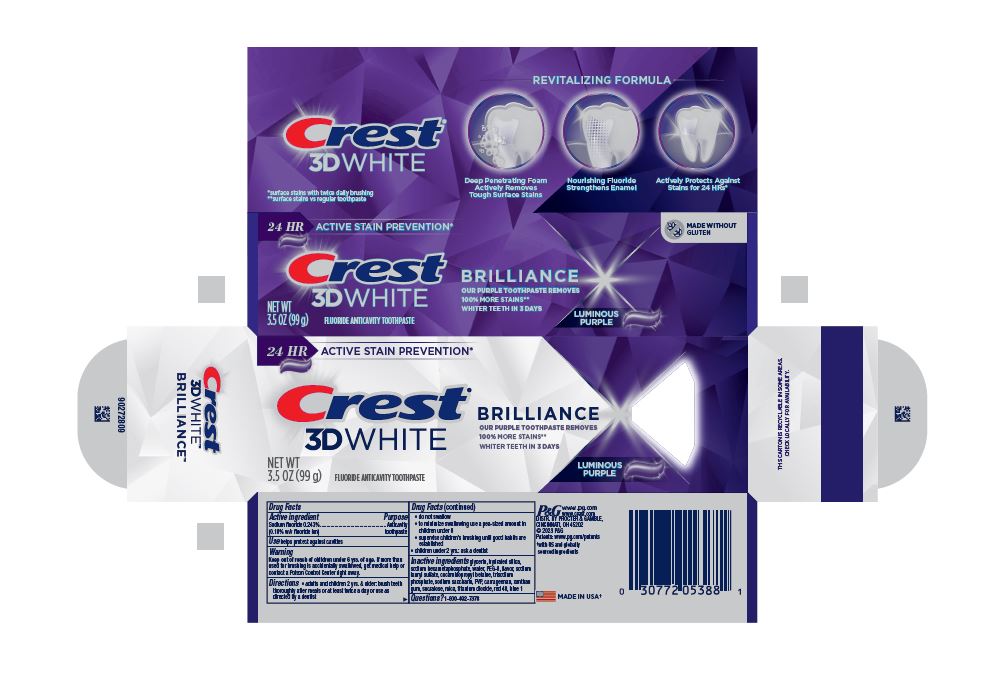
 24 HR ACTIVE STAIN PREVENTION*
24 HR ACTIVE STAIN PREVENTION*