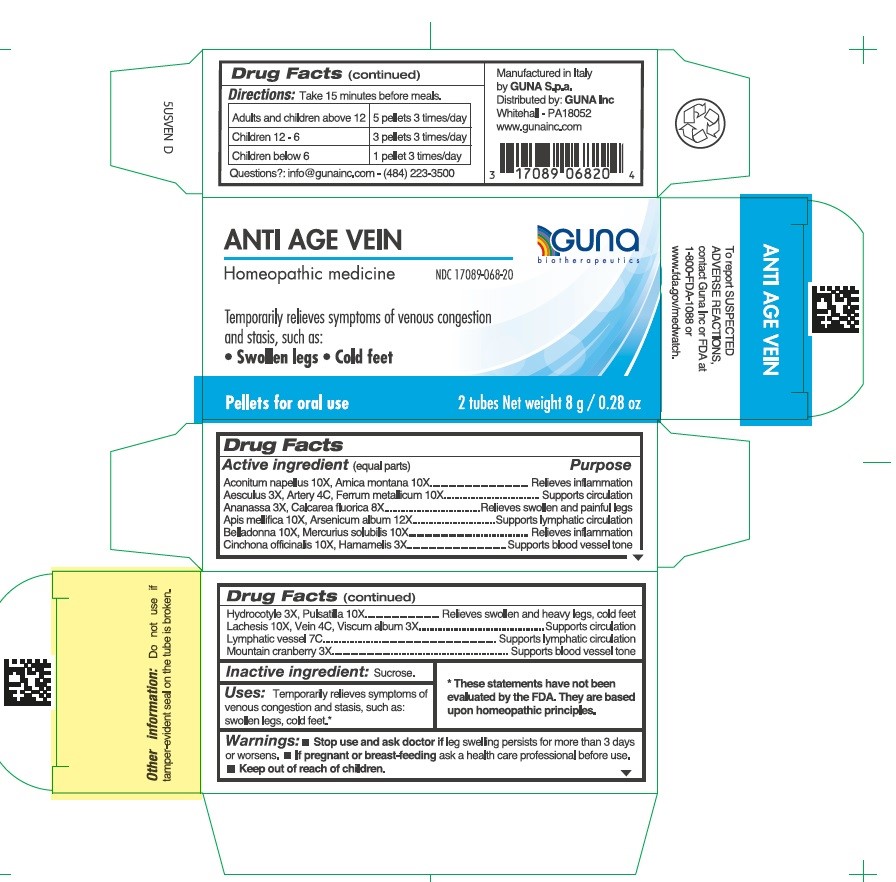
Label: ANTI AGE VEIN- aconitum napellus - apis mellifera - arnica montana - arsenic trioxide - atropa belladonna - calcium fluoride - centella asiatica - cinchona officinalis bark - cranberry - horse chestnut - iron - lachesis muta venom - mercury - pineapple - pulsatilla vulgaris - sus scrofa artery - sus scrofa small intestine mucosa lymph follicle - sus scrofa vein - viscum album fruit - witch hazel - pellet
- NDC Code(s): 17089-068-20
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Aconitum napellus 10X, Arnica montana 10X.....................................Relieves inflammation
Aesculus hippocastanum 3X, Artery 4C, Ferrum metallicum 10X.......Supports circulation
Ananassa 3X, Calcarea fluorica 8X.....................................................Relieves swollen and painful legs
Apis mellifica 10X, Arsenicum album 12X...........................................Supports lymphatic circulation
Belladonna 10X, Mercurius solubilis 10X............................................Relieves inflammation
Cinchona officinalis 10X, Hamamelis 3X.............................................Supports blood vessel tone
Hydrocotyle 3X, Pulsatilla 10X............................................................Relieves swollen and heavy legs, cold feet
Lachesis 10X, Vein 4C, Viscum album 3X..........................................Supports circulation
Lymphatic vessel 7C...........................................................................Supports lymphatic circulation
Mountain cranberry 3X........................................................................Supports blood vessel tone
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- QUESTIONS
- Directions
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI AGE VEIN
aconitum napellus - apis mellifera - arnica montana - arsenic trioxide - atropa belladonna - calcium fluoride - centella asiatica - cinchona officinalis bark - cranberry - horse chestnut - iron - lachesis muta venom - mercury - pineapple - pulsatilla vulgaris - sus scrofa artery - sus scrofa small intestine mucosa lymph follicle - sus scrofa vein - viscum album fruit - witch hazel - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-068 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 10 [hp_X] in 4 g HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 3 [hp_X] in 4 g PINEAPPLE (UNII: 2A88ZO081O) (PINEAPPLE - UNII:2A88ZO081O) PINEAPPLE 3 [hp_X] in 4 g APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 10 [hp_X] in 4 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 10 [hp_X] in 4 g ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_C] in 4 g SUS SCROFA ARTERY (UNII: 63O327782Q) (SUS SCROFA ARTERY - UNII:63O327782Q) SUS SCROFA ARTERY 4 [hp_C] in 4 g ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 10 [hp_X] in 4 g CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 8 [hp_X] in 4 g CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 4 g IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 10 [hp_X] in 4 g WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 3 [hp_X] in 4 g CENTELLA ASIATICA (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA 3 [hp_X] in 4 g LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 10 [hp_X] in 4 g SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE (UNII: 308LM01C72) (SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE - UNII:308LM01C72) SUS SCROFA SMALL INTESTINE MUCOSA LYMPH FOLLICLE 7 [hp_C] in 4 g MERCURY (UNII: FXS1BY2PGL) (MERCURY - UNII:FXS1BY2PGL) MERCURY 10 [hp_X] in 4 g CRANBERRY (UNII: 0MVO31Q3QS) (CRANBERRY - UNII:0MVO31Q3QS) CRANBERRY 3 [hp_X] in 4 g PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 10 [hp_X] in 4 g SUS SCROFA VEIN (UNII: 2510RH3I89) (SUS SCROFA VEIN - UNII:2510RH3I89) SUS SCROFA VEIN 4 [hp_C] in 4 g VISCUM ALBUM FRUIT (UNII: P83EQ521R3) (VISCUM ALBUM FRUIT - UNII:P83EQ521R3) VISCUM ALBUM FRUIT 3 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 3.5 g in 4 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-068-20 2 in 1 BOX 12/21/2018 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-068)