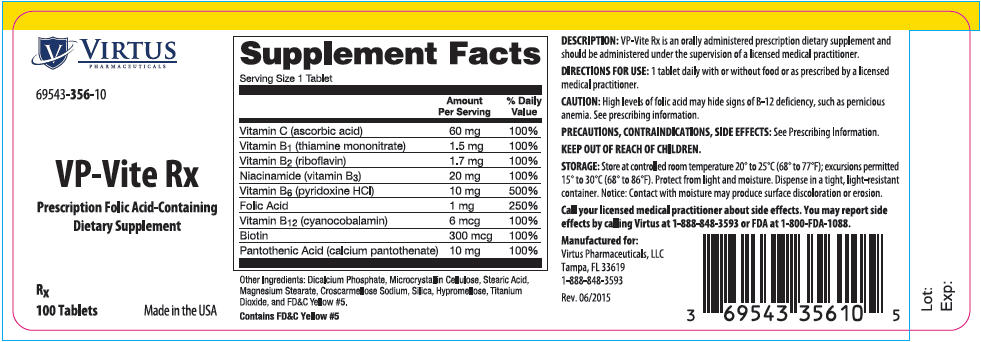
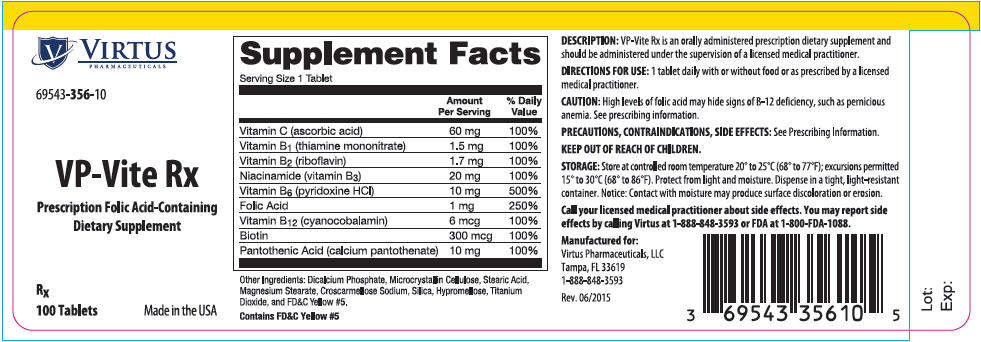
Label: VP-VITE RX- ascorbic acid, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, and calcium pantothenate tablet
- NHRIC Code(s): 69543-356-10
- Packager: Virtus Pharmaceuticals
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated September 17, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CLAIM
-
DESCRIPTION
VP-Vite Rx is an orally administered prescription dietary supplement and should be administered under the supervision of a licensed medical practitioner.
Supplement Facts Serving Size: 1 Tablet Amount Per Serving % Daily Value Vitamin C
(ascorbic acid)60 mg 100% Vitamin B1
(thiamine mononitrate)1.5 mg 100% Vitamin B2
(riboflavin)1.7 mg 100% Niacinamide
(vitamin B3)20 mg 100% Vitamin B6
(pyridoxine HCl)10 mg 500% Folic Acid 1 mg 250% Vitamin B12
(cyanocobalamin)6 mcg 100% Biotin 300 mcg 100% Pantothenic Acid
(calcium pantothenate)10 mg 100% Other Ingredients: Dicalcium Phosphate, Microcrystalline Cellulose, Stearic Acid, Magnesium Stearate, Croscarmellose Sodium, Silica, Hypromellose, Titanium Dioxide, and FD&C Yellow #5.
Contains FD&C Yellow #5
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- PATIENT INFORMATION
-
DRUG INTERACTIONS
Pyridoxine hydrochloride should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
Drugs which may interact with folate include:
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate. Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the levels of this AED in the blood and allowing breakthrough seizures to occur.
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs including aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim, block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels.
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of 5-methyltetrahydrofolate active transport in the intestine.
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs, including ibuprofen, naproxen, indomethacin and sulindac, have been shown to inhibit some folate-dependent enzymes in laboratory experiments.
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- Pancreatic Enzymes: Reduced folate levels have occurred in some patients taking pancreatic extracts.
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine treatment.
- Smoking and alcohol: Reduced serum folate levels have been noted.
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
-
SIDE EFFECTS
Allergic sensitization has been reported following oral administration of folic acid. Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with cyanocobalamin.
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
VP-VITE RX
ascorbic acid, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, and calcium pantothenate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69543-356 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 60 mg Thiamine Mononitrate (UNII: 8K0I04919X) (Thiamine Ion - UNII:4ABT0J945J) Thiamine 1.5 mg Riboflavin (UNII: TLM2976OFR) (Riboflavin - UNII:TLM2976OFR) Riboflavin 1.7 mg Niacinamide (UNII: 25X51I8RD4) (Niacinamide - UNII:25X51I8RD4) Niacinamide 20 mg Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (Pyridoxine - UNII:KV2JZ1BI6Z) Pyridoxine 10 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 1 mg Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 6 ug Biotin (UNII: 6SO6U10H04) (Biotin - UNII:6SO6U10H04) Biotin 300 ug Calcium Pantothenate (UNII: 568ET80C3D) (Pantothenic Acid - UNII:19F5HK2737, Calcium Cation - UNII:2M83C4R6ZB) Pantothenic Acid 10 mg Inactive Ingredients Ingredient Name Strength Calcium Phosphate, Dibasic, Anhydrous (UNII: L11K75P92J) Cellulose, Microcrystalline (UNII: OP1R32D61U) Stearic Acid (UNII: 4ELV7Z65AP) Magnesium Stearate (UNII: 70097M6I30) Croscarmellose Sodium (UNII: M28OL1HH48) Silicon Dioxide (UNII: ETJ7Z6XBU4) Hypromelloses (UNII: 3NXW29V3WO) Titanium Dioxide (UNII: 15FIX9V2JP) FD&C Yellow No. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69543-356-10 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 09/08/2015 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 10 mm imprint Labeler - Virtus Pharmaceuticals (079659493)