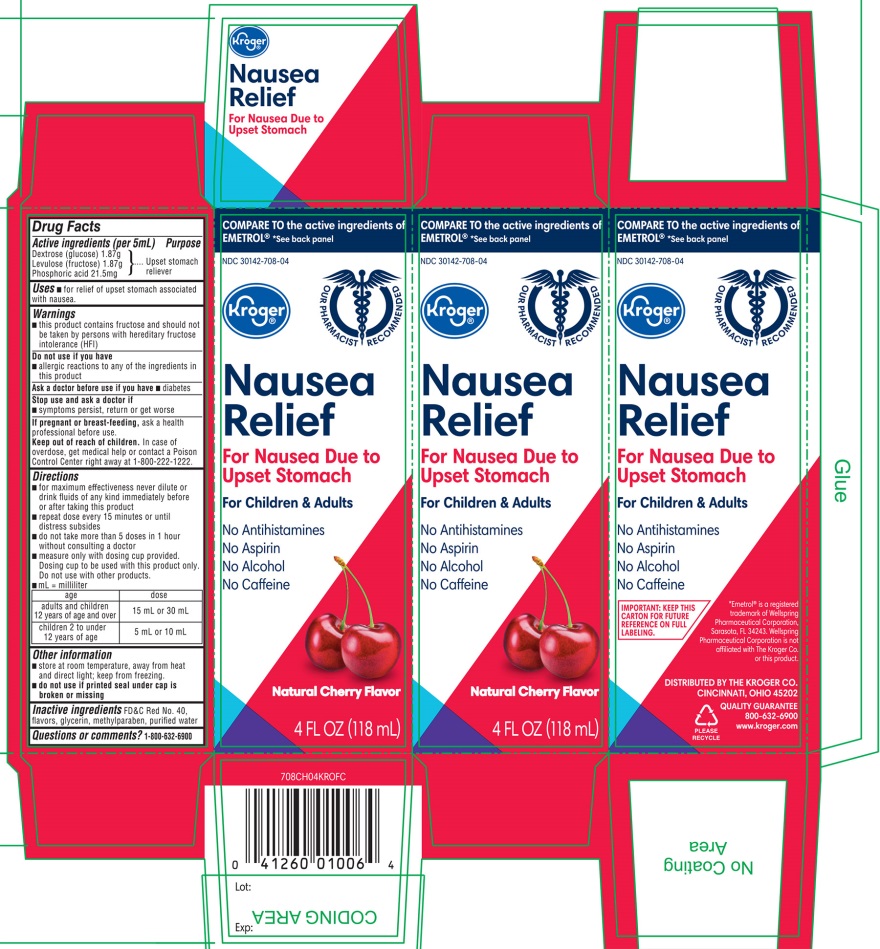
Label: KROGER ANTI NAUSEA CHERRY FLAVOR (dextrose(glucose),levulose- fructose,phosphoric acid liquid
- NDC Code(s): 30142-708-04
- Packager: KROGER COMPANY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (per 5 mL)
- Purpose
- Uses
- Warnings
-
Directions
- ▪
- for maximum effectiveness never dilute or drink fluids of any kind immediately before or after taking this product
- ▪
- repeat dose every 15 minutes or until distress subsides
- ▪
- do not take more than 5 doses in 1 hour without consulting a doctor
- ▪
- measure only with dosing cup provided. Dosing cup to be used with this product only. Do not use with other products.
- ▪
- mL= milliliter
- Age
- dose
- adults and children 12 years of age and over
- 15 mL or 30 mL
- children 2 to under 12 years of age
- 5 ml or 10 mL
- Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
NDC 30142-708-04
COMPARE TO the active ingredients EMETROL® * See back panel
Kroger®
Nausea Relief
For Nausea Due to Upset Stomach
For Children & Adults
- •
- No Antihistamines
- •
- No Aspirin
- •
- No Alcohol
- •
- No caffeine
Naturally Cherry Flavor
Naturally and Artificially Flavored
4 FL OZ (118 mL)
IMPORTANT: KEEP THIS CARTON FOR FUTURE REFERENCE ON FULL LABELING.
*Emetrol® is a registered trademark of Wellspring Pharmaceuticals Corporation, Sarasota, FL 34243. Wellspring Pharmaceuticals Corporation is not affiliated with The Kroger Co. or this product.
DISTRIBUTED BY THE KROGER CO.
CINCINNATI, OHIO 45202
QUALITY GUARANTEE
800-632-6900
www.kroger.com
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
KROGER ANTI NAUSEA CHERRY FLAVOR
dextrose(glucose),levulose(fructose),phosphoric acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-708 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) (DEXTROSE, UNSPECIFIED FORM - UNII:IY9XDZ35W2) DEXTROSE, UNSPECIFIED FORM 1.87 g in 5 mL FRUCTOSE (UNII: 6YSS42VSEV) (FRUCTOSE - UNII:6YSS42VSEV) FRUCTOSE 1.87 g in 5 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 21.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) Product Characteristics Color RED (Red) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-708-04 1 in 1 CARTON 03/31/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 03/31/2020 Labeler - KROGER COMPANY (006999528)