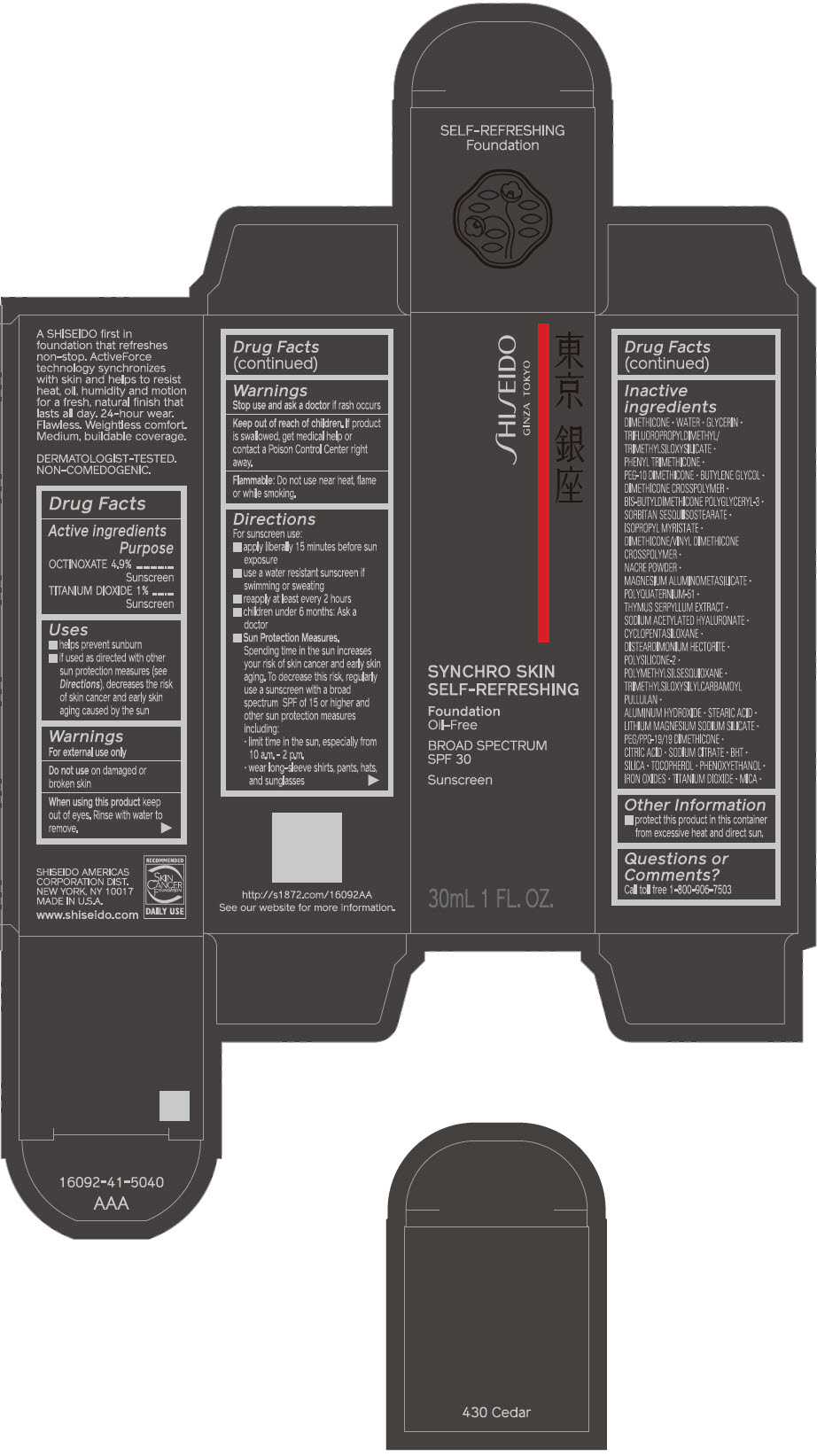
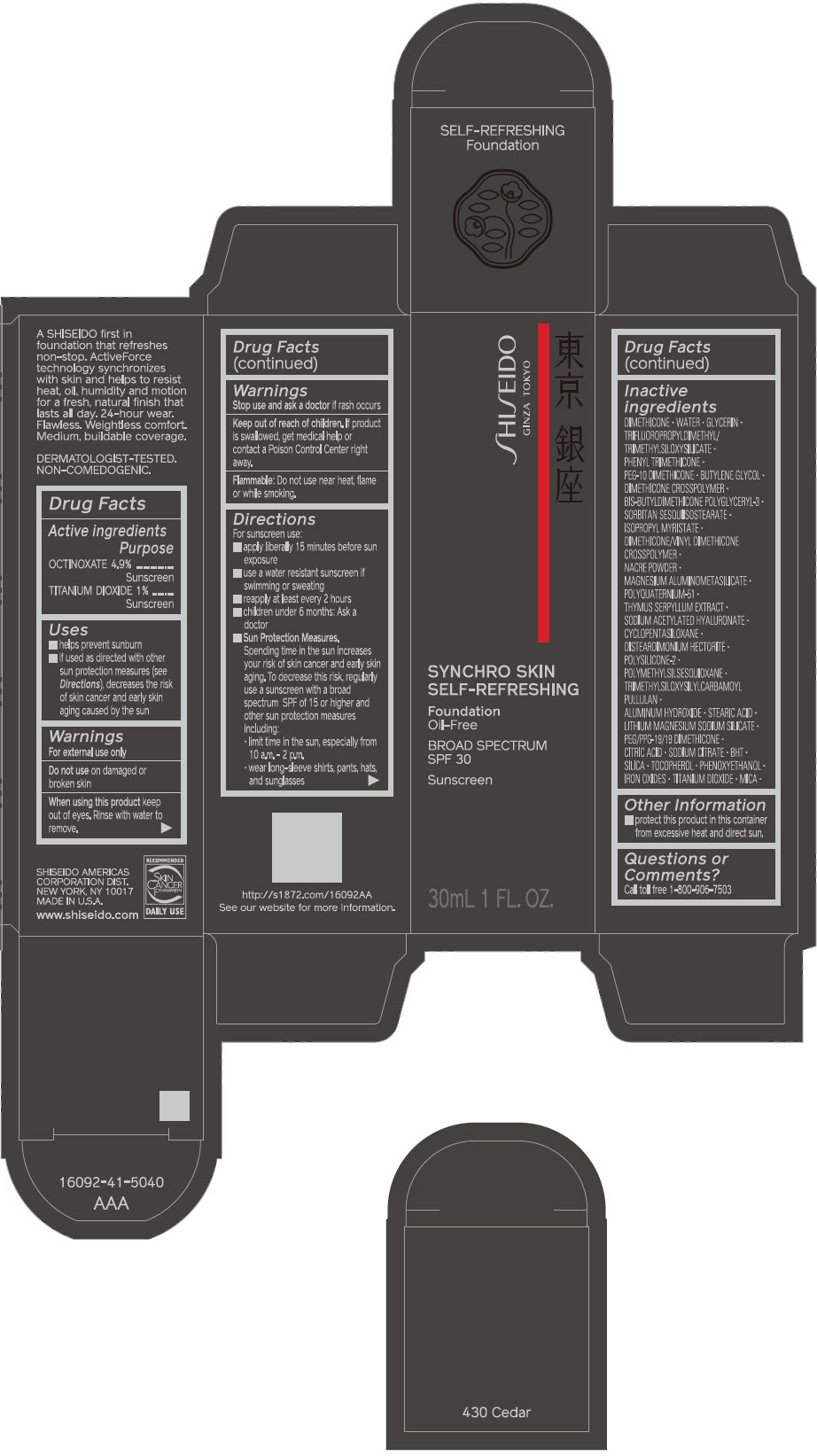
Label: SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 430 CEDAR- octinoxate and titanium dioxide emulsion
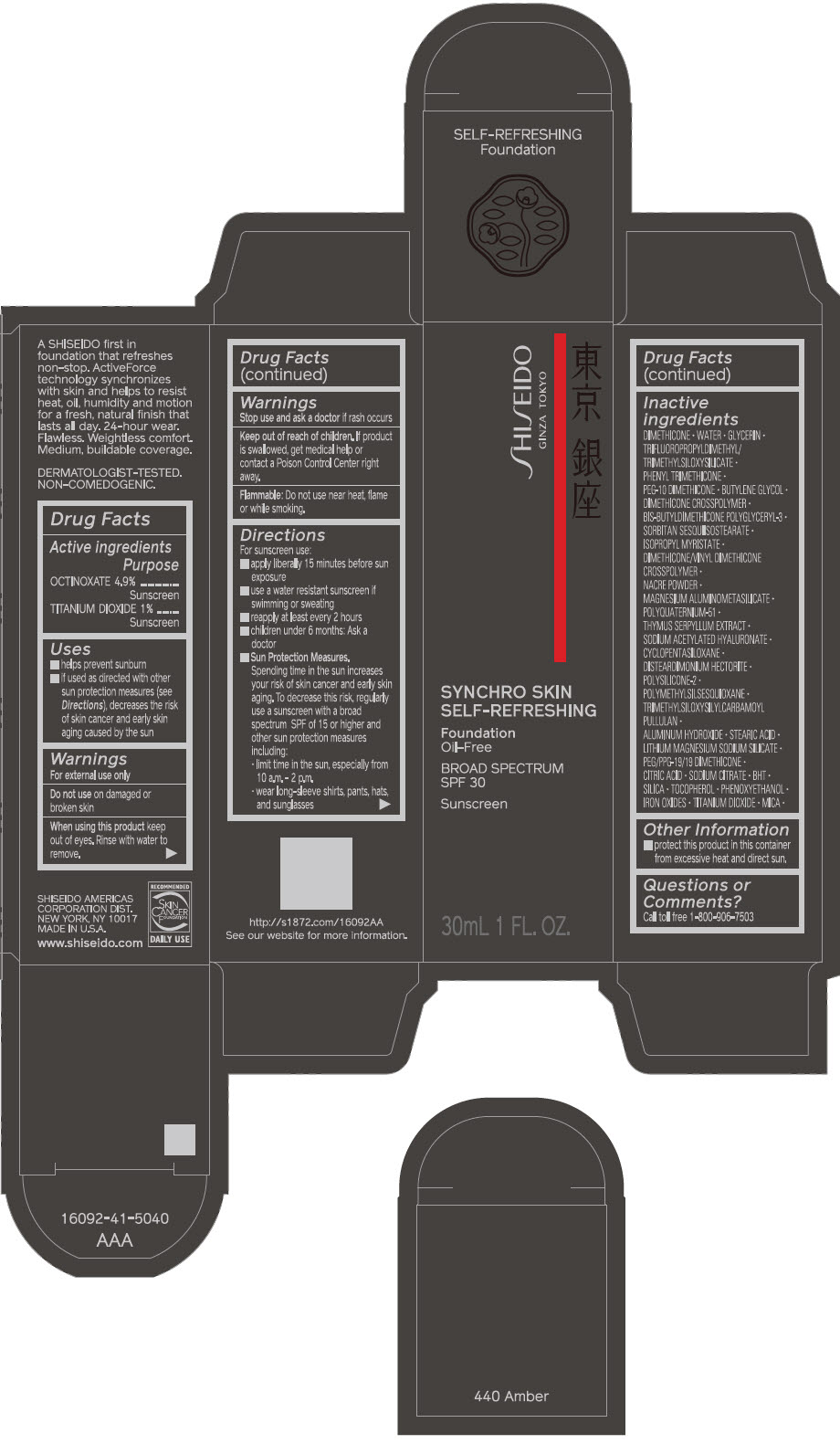
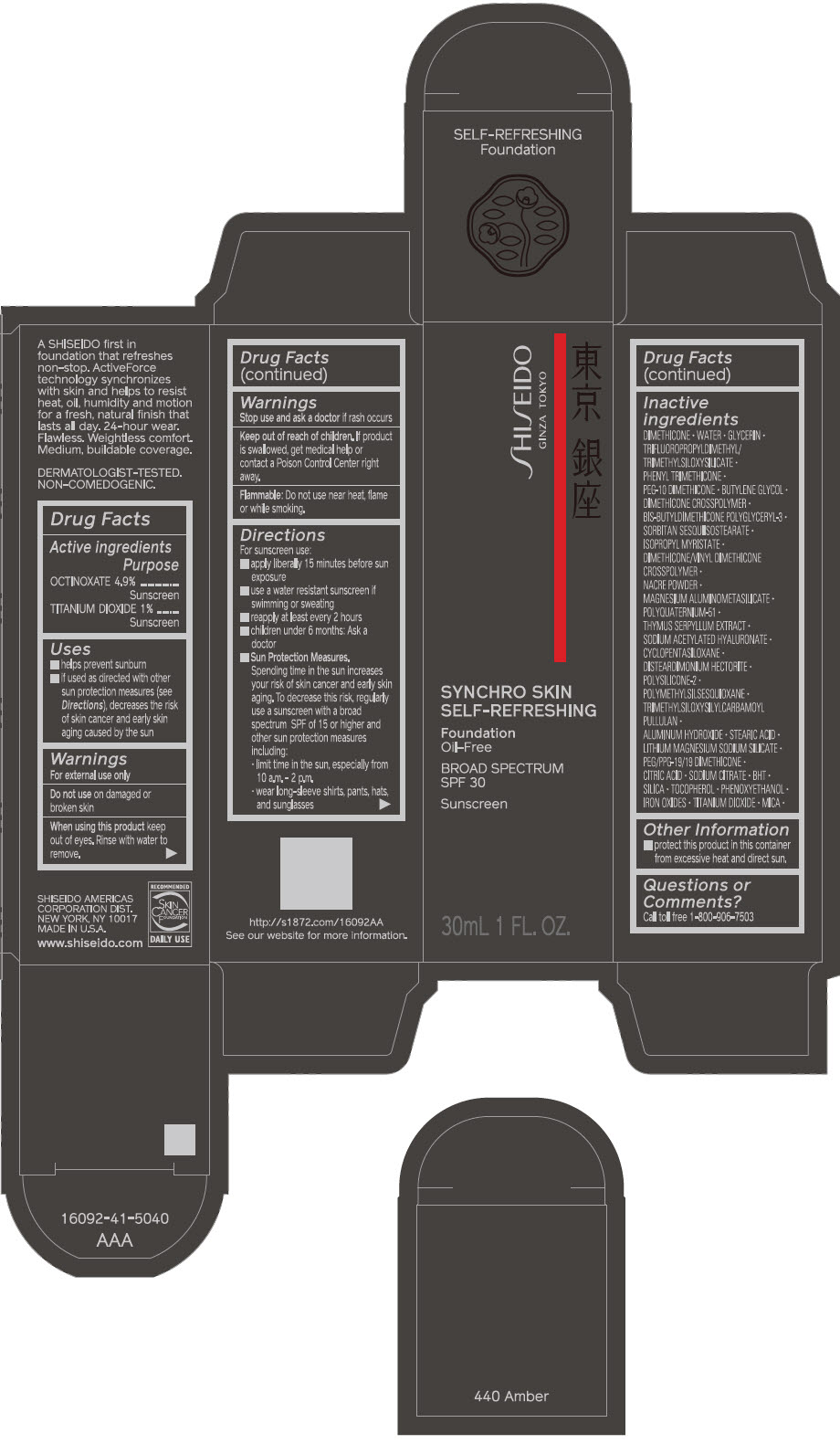
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 440 AMBER- octinoxate and titanium dioxide emulsion
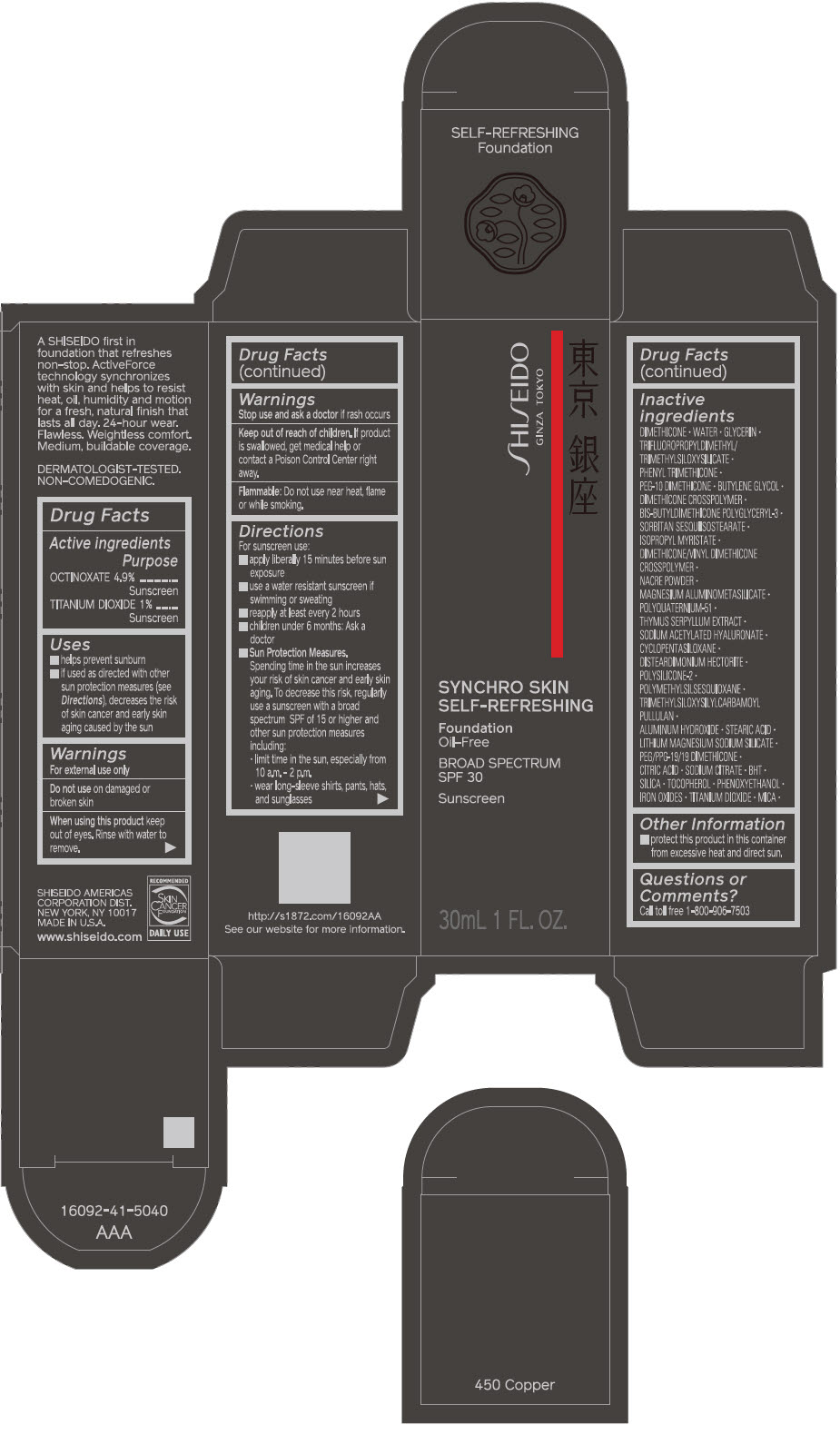
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 450 COPPER- octinoxate and titanium dioxide emulsion
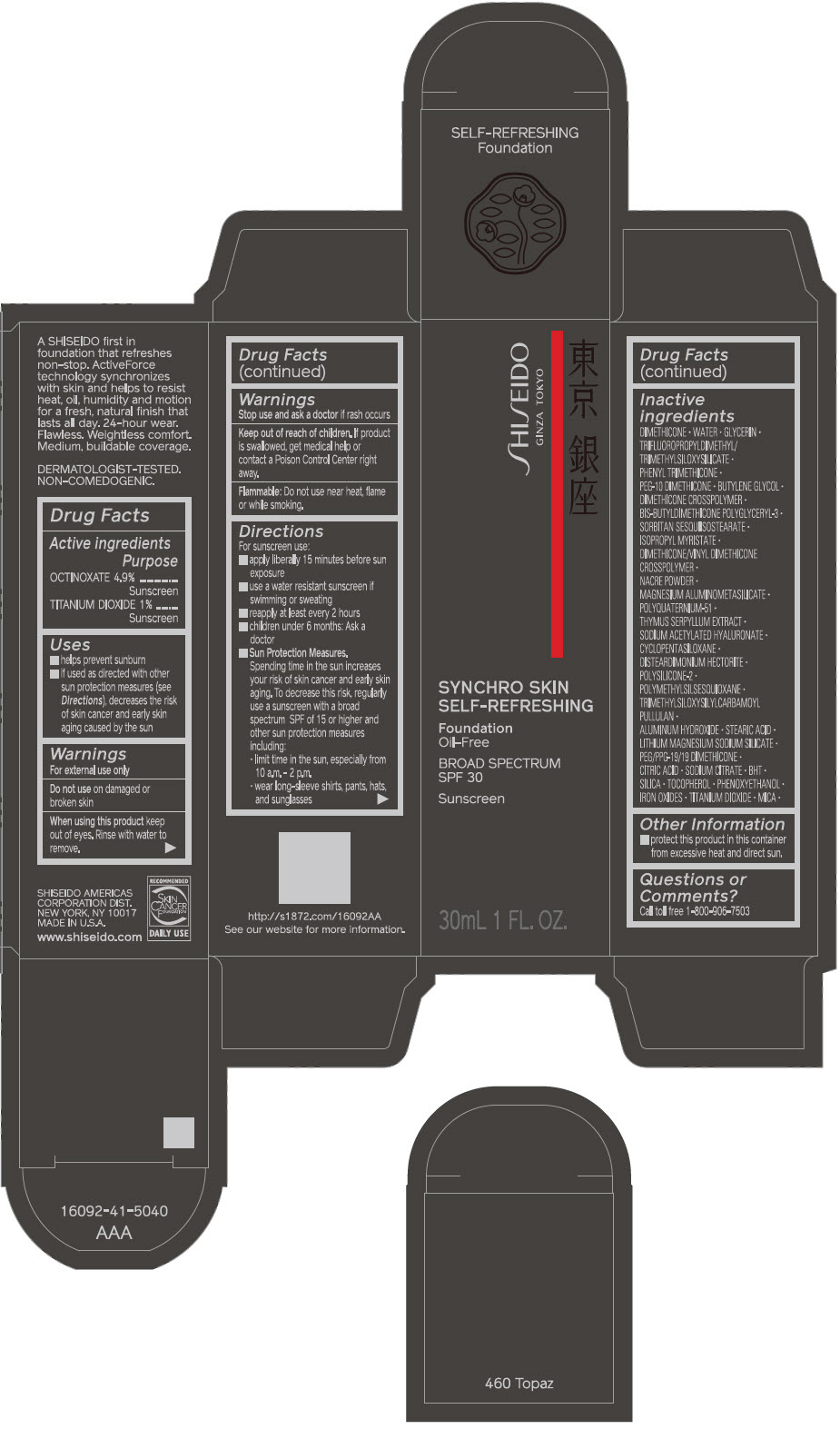
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 460 TOPAZ- octinoxate and titanium dioxide emulsion
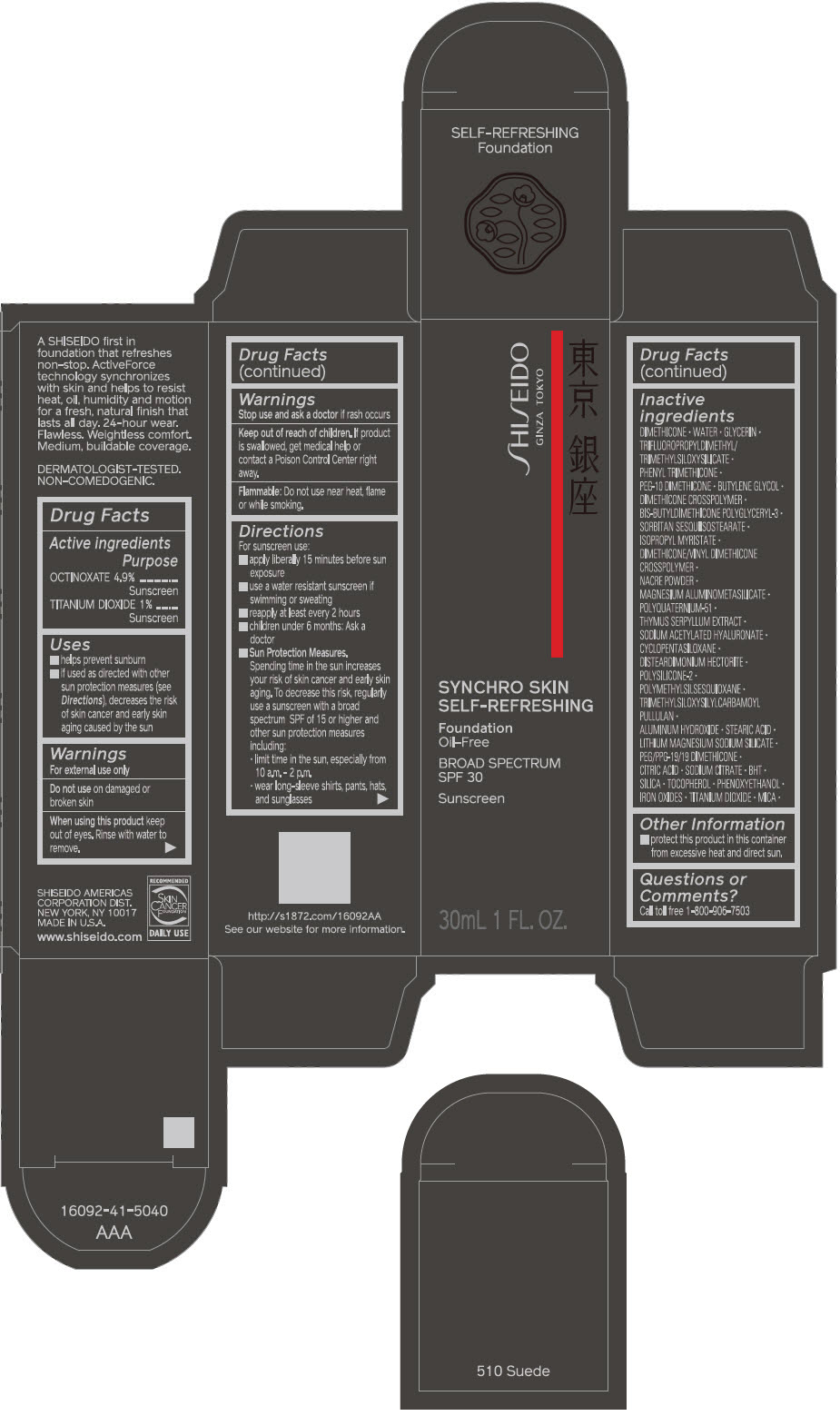
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 510 SUEDE- octinoxate and titanium dioxide emulsion
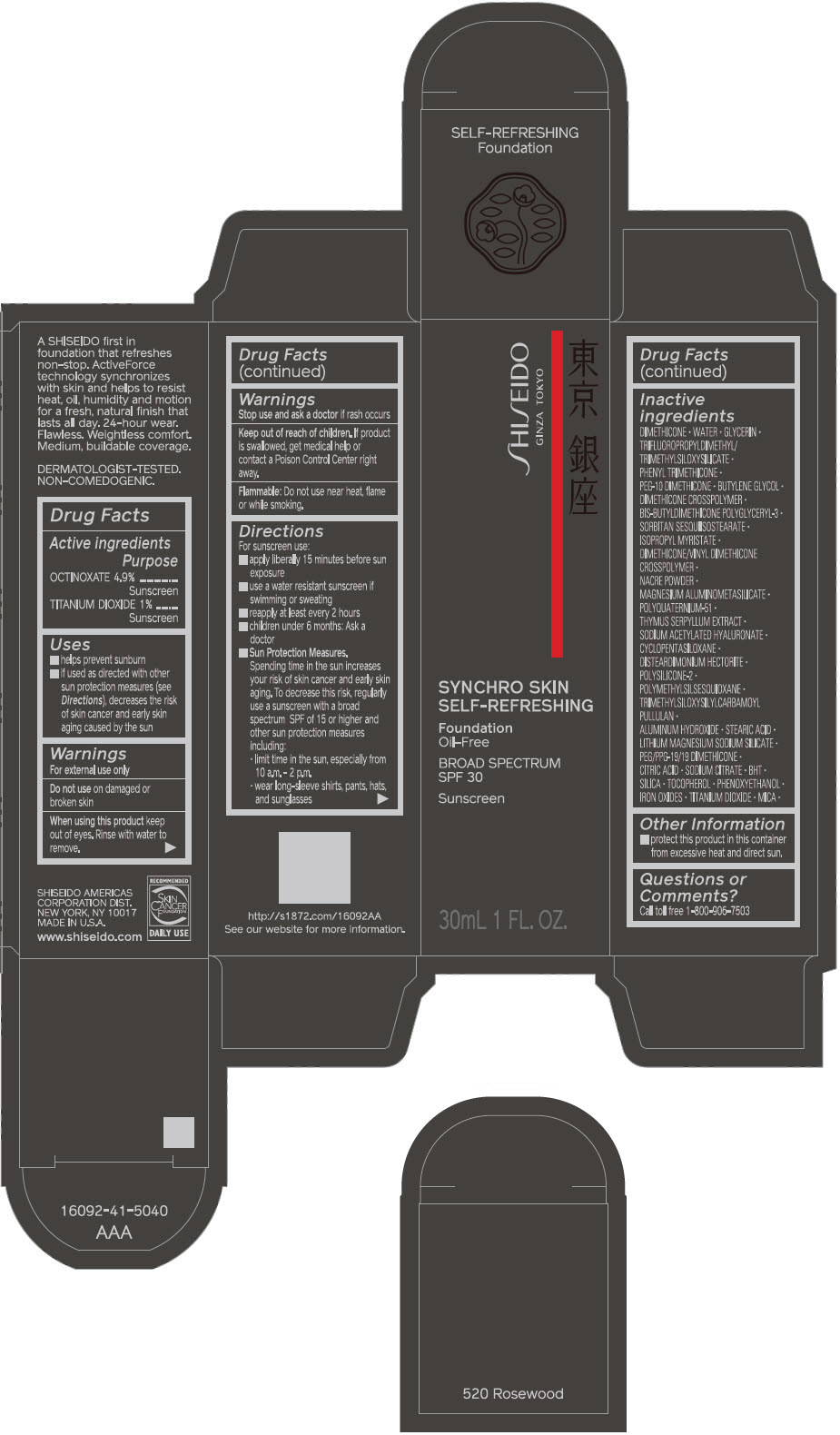
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 520 ROSEWOOD- octinoxate and titanium dioxide emulsion
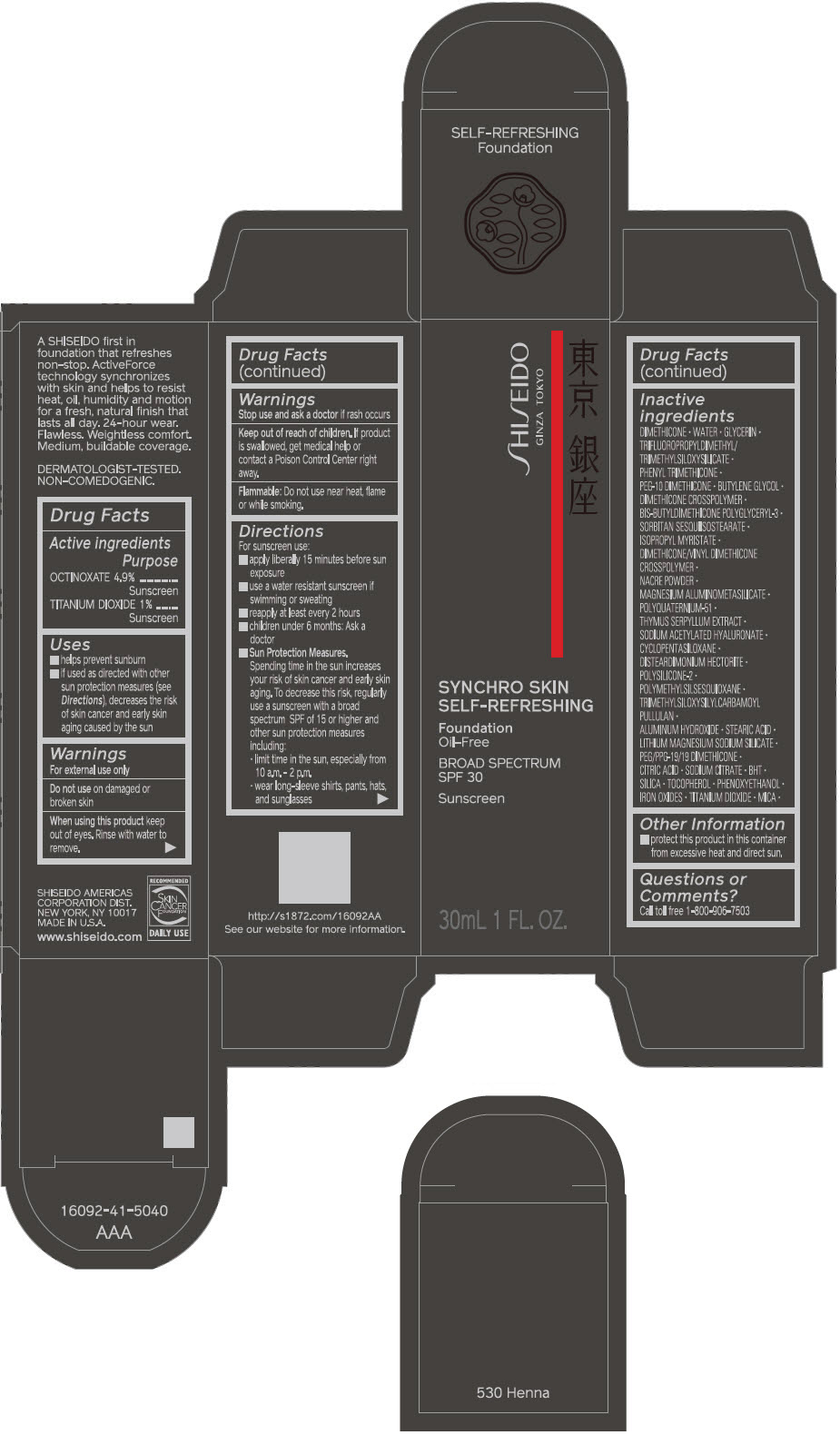
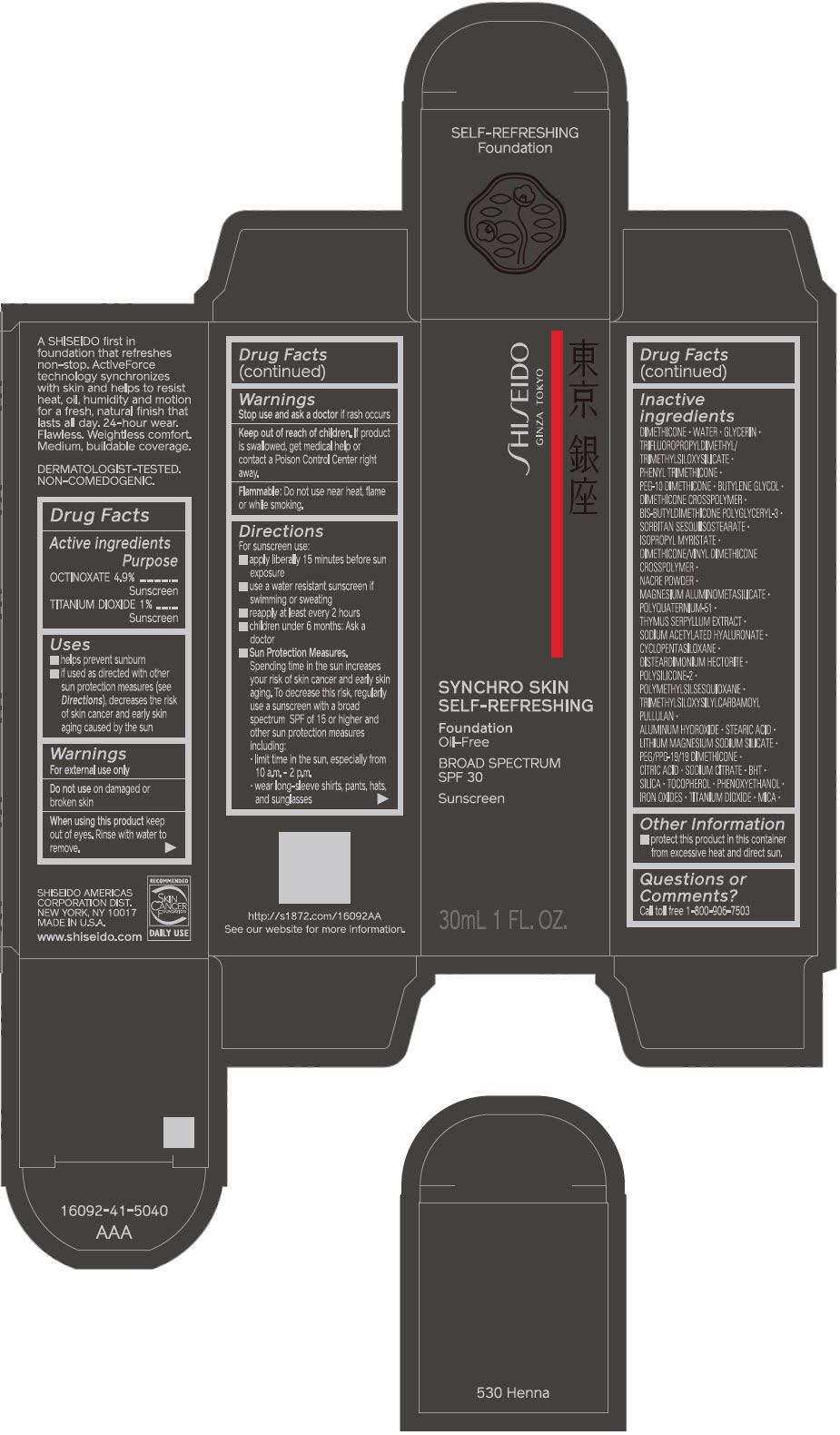
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 530 HENNA- octinoxate and titanium dioxide emulsion
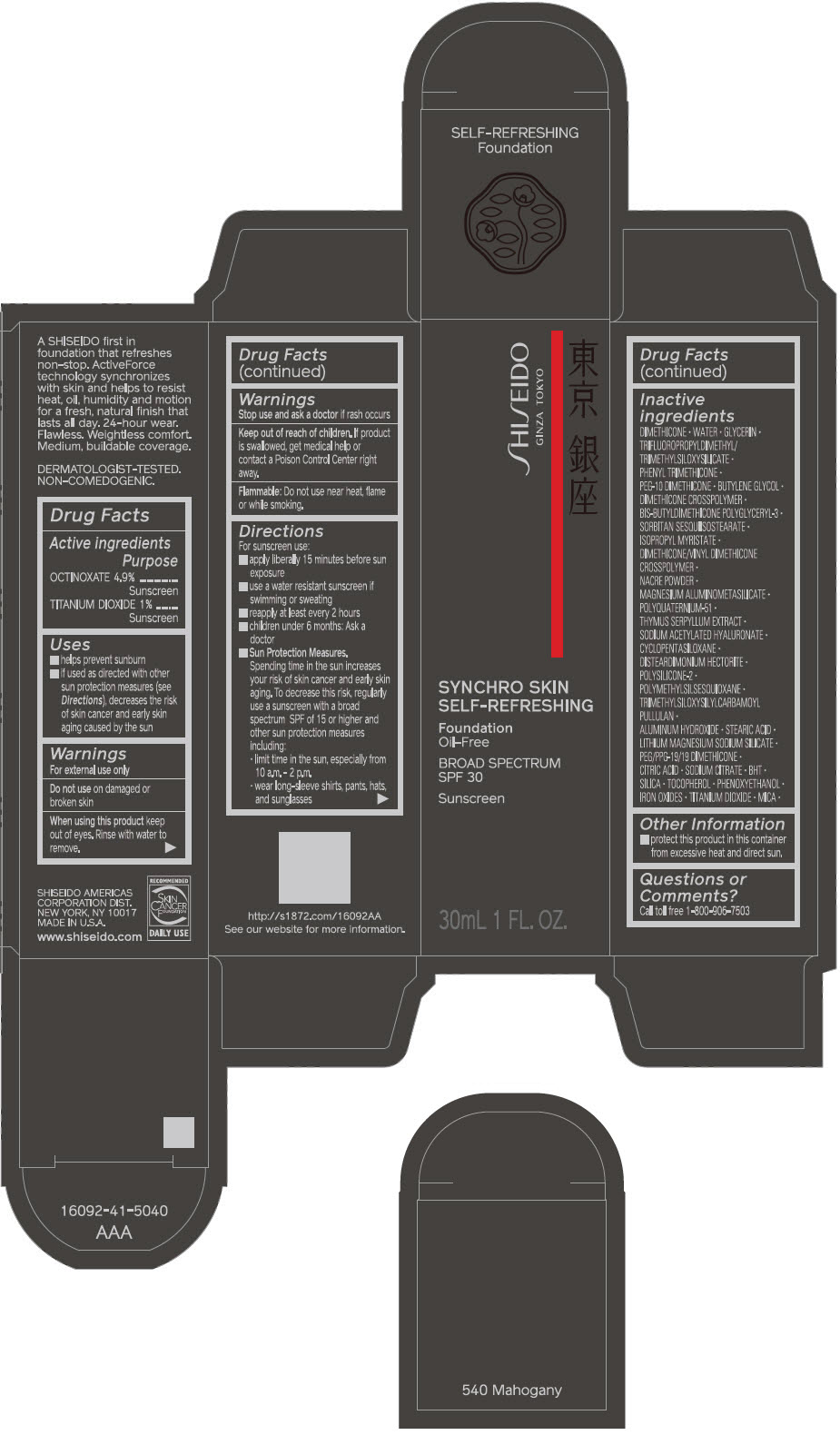
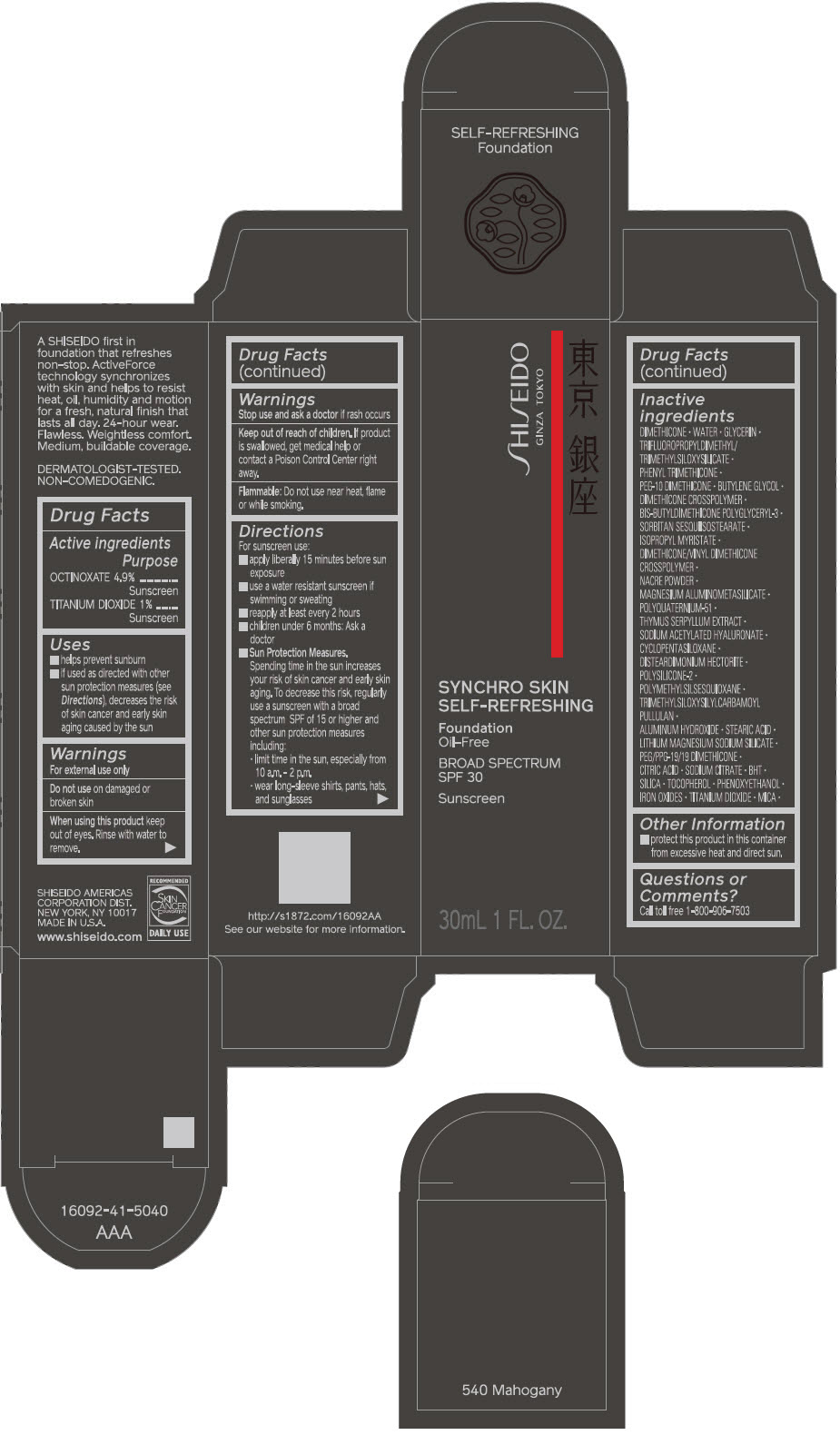
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 540 MAHOGANY- octinoxate and titanium dioxide emulsion
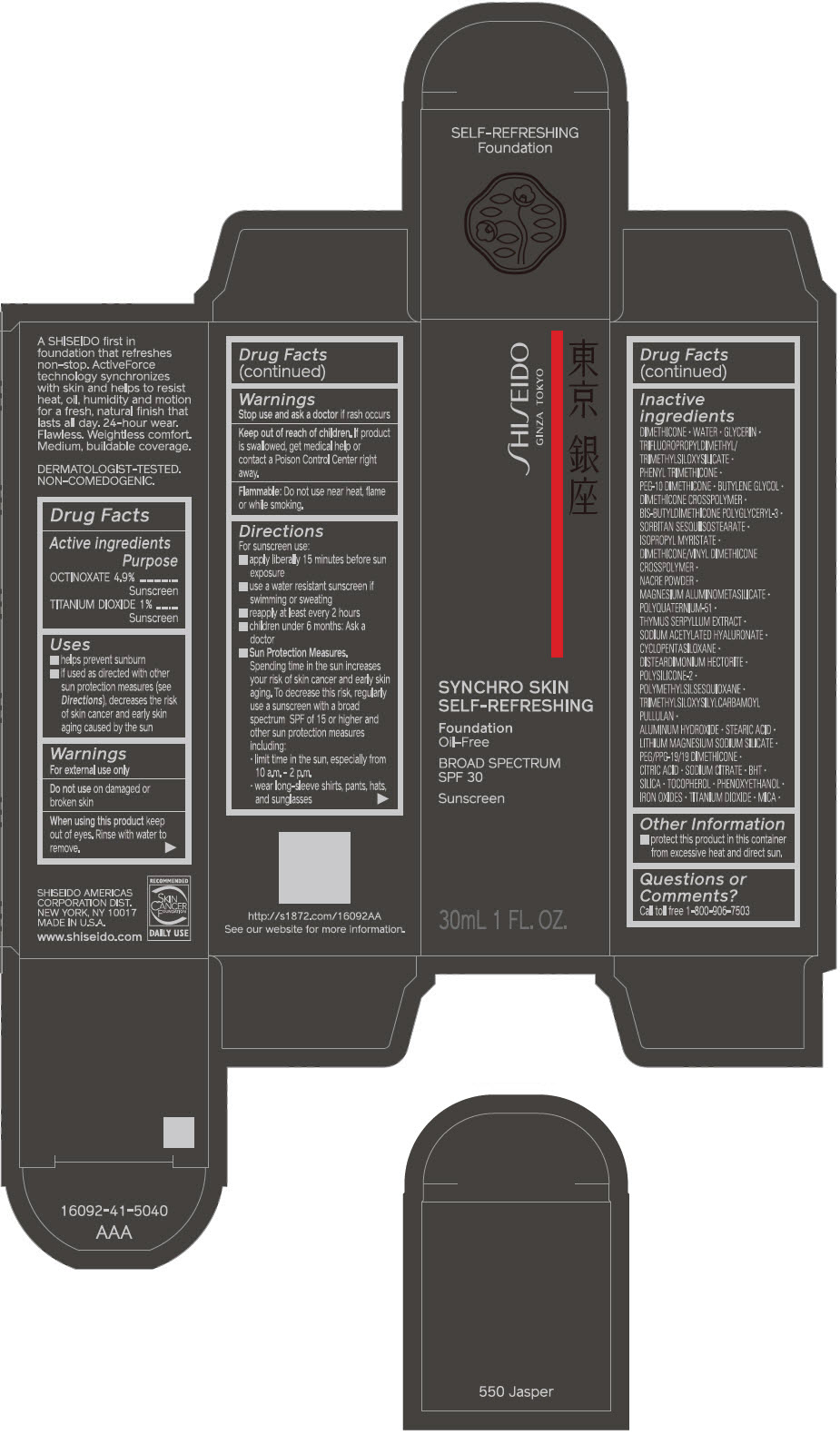
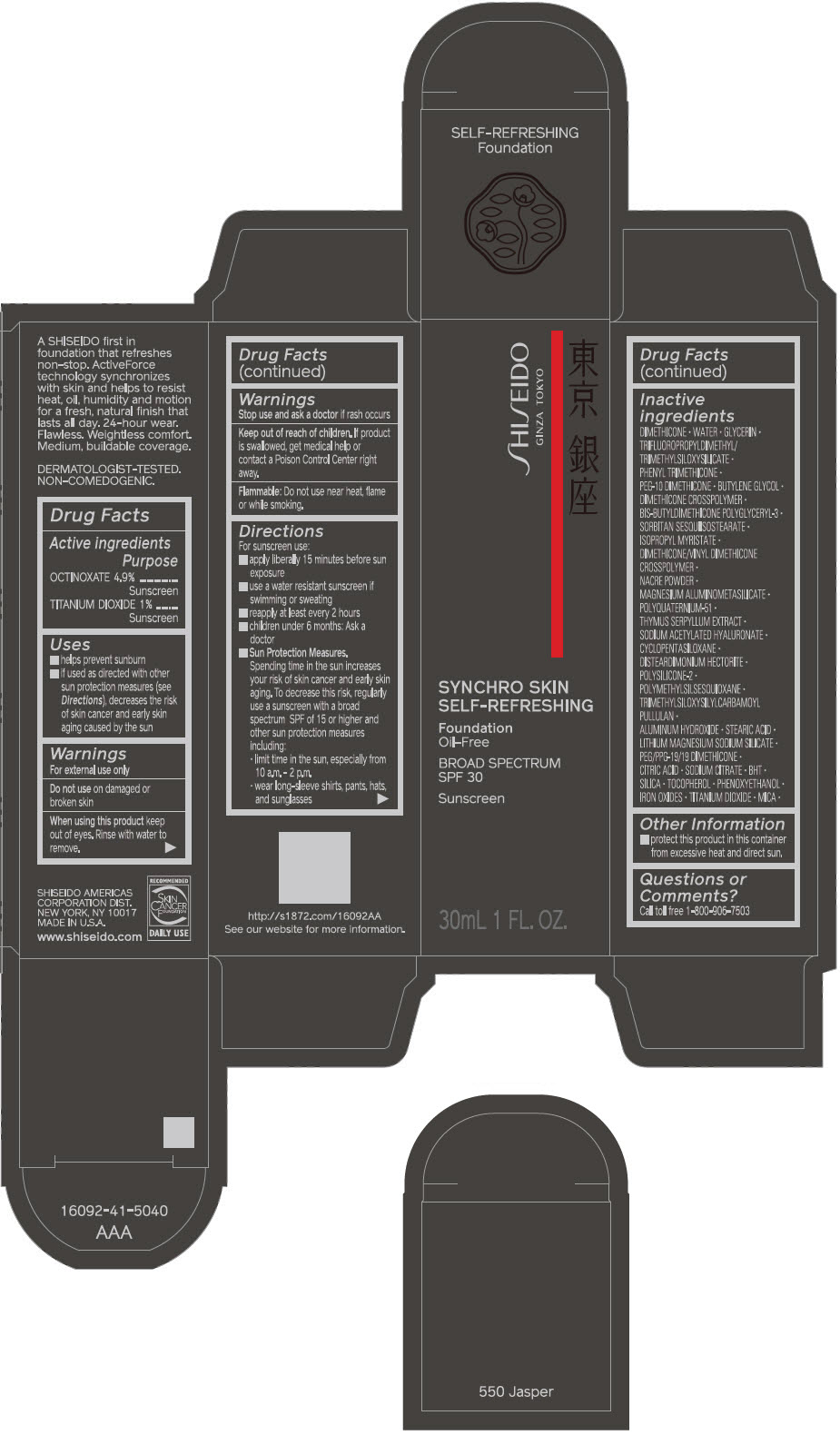
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 550 JASPER- octinoxate and titanium dioxide emulsion
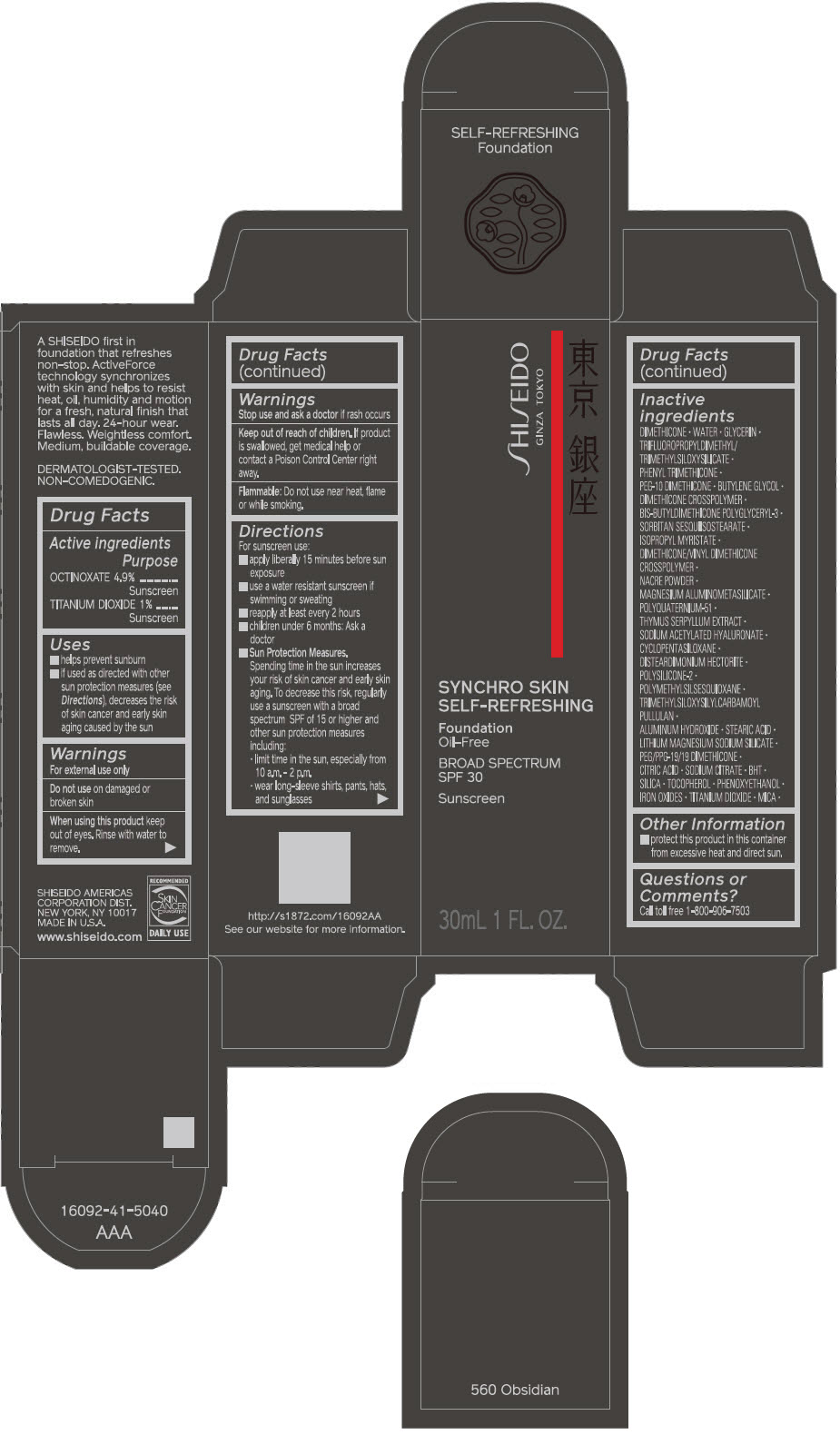
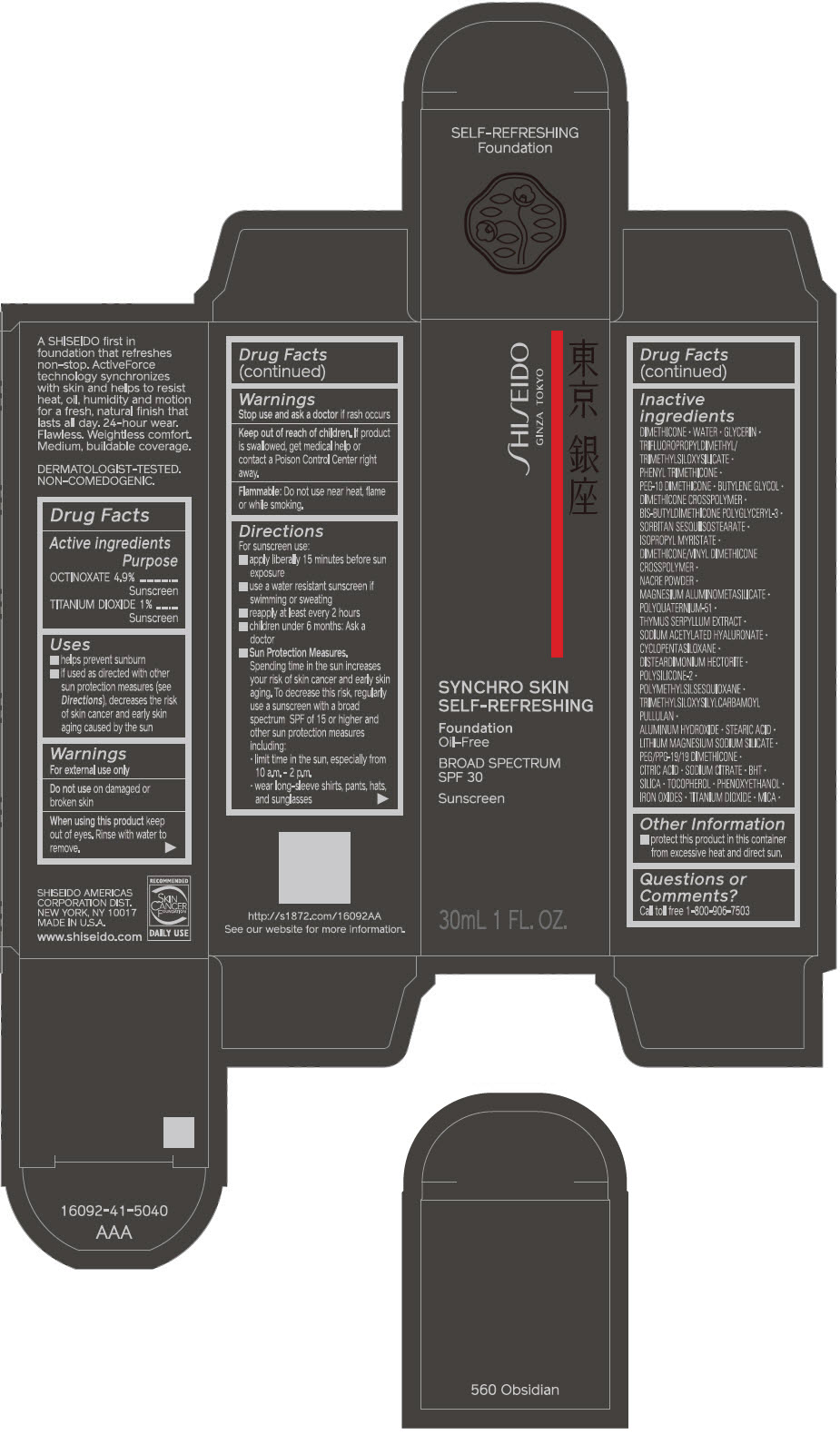
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 560 OBSIDIAN- octinoxate and titanium dioxide emulsion
-
NDC Code(s):
58411-504-10,
58411-505-10,
58411-506-10,
58411-507-10, view more58411-508-10, 58411-509-10, 58411-510-10, 58411-511-10, 58411-512-10, 58411-513-10
- Packager: SHISEIDO AMERICAS CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
Inactive Ingredients
DIMETHICONE • WATER • GLYCERIN • TRIFLUOROPROPYLDIMETHYL/TRIMETHYLSILOXYSILICATE • PHENYL TRIMETHICONE • PEG-10 DIMETHICONE • BUTYLENE GLYCOL • DIMETHICONE CROSSPOLYMER • BIS-BUTYLDIMETHICONE POLYGLYCERYL-3 • SORBITAN SESQUIISOSTEARATE • ISOPROPYL MYRISTATE • DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER • NACRE POWDER • MAGNESIUM ALUMINOMETASILICATE • POLYQUATERNIUM-51 • THYMUS SERPYLLUM EXTRACT • SODIUM ACETYLATED HYALURONATE • CYCLOPENTASILOXANE • DISTEARDIMONIUM HECTORITE • POLYSILICONE-2 • POLYMETHYLSILSESQUIOXANE • TRIMETHYLSILOXYSILYLCARBAMOYL PULLULAN • ALUMINUM HYDROXIDE • STEARIC ACID • LITHIUM MAGNESIUM SODIUM SILICATE • PEG/PPG-19/19 DIMETHICONE • CITRIC ACID • SODIUM CITRATE • BHT • SILICA • TOCOPHEROL • PHENOXYETHANOL • IRON OXIDES • TITANIUM DIOXIDE • MICA •
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 430 Cedar
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 440 Amber
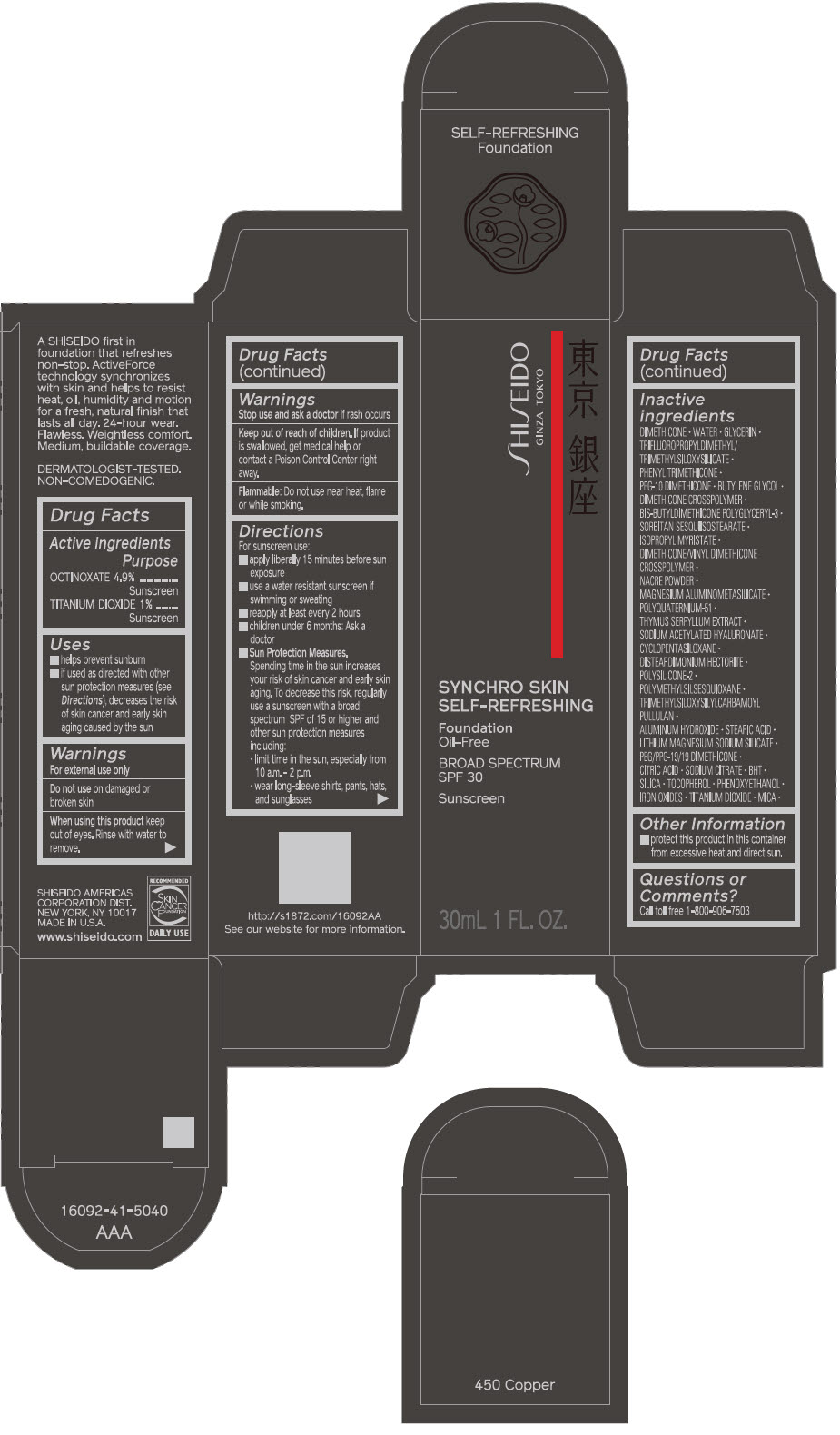
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 450 Copper
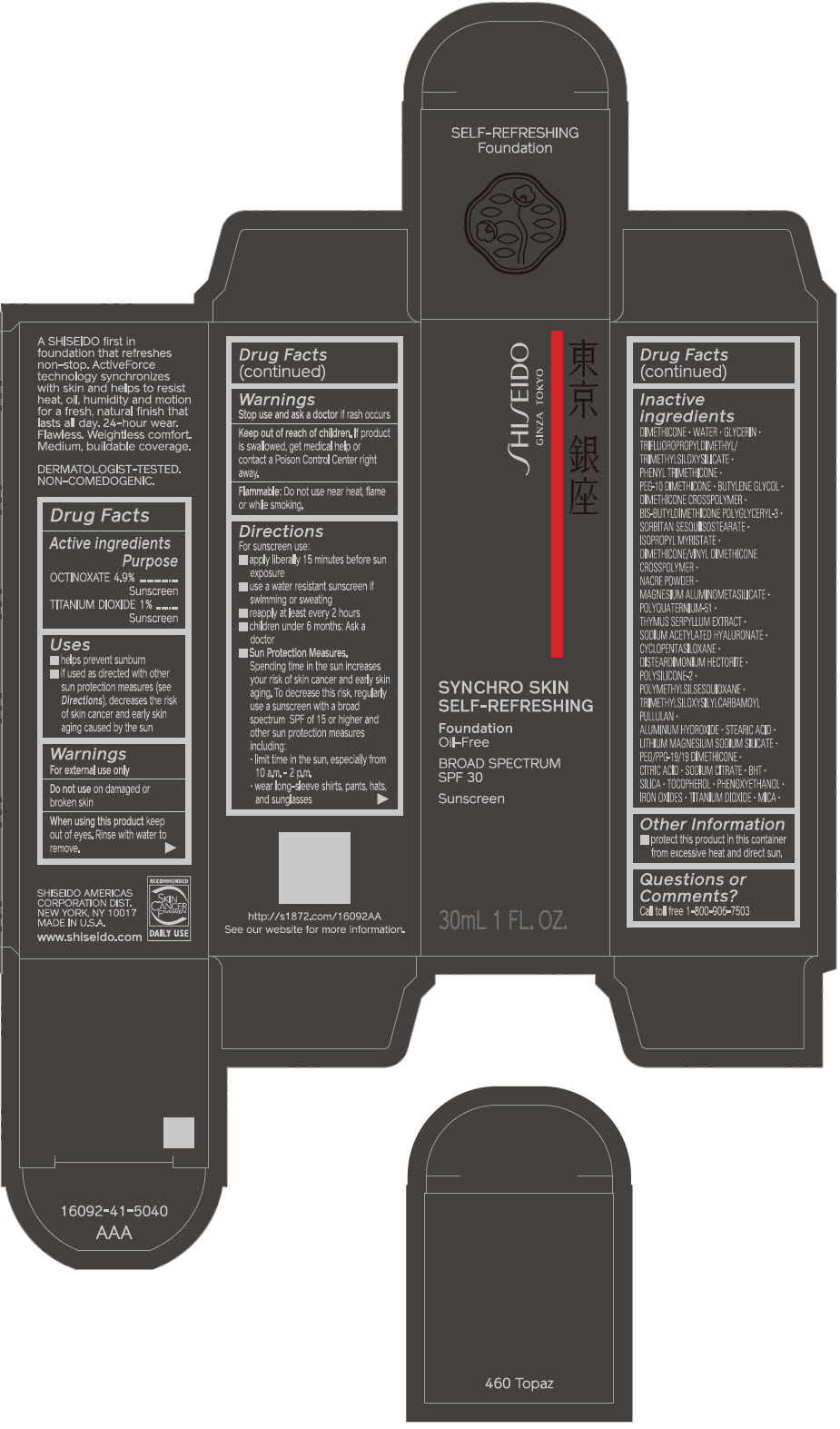
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 460 Topaz
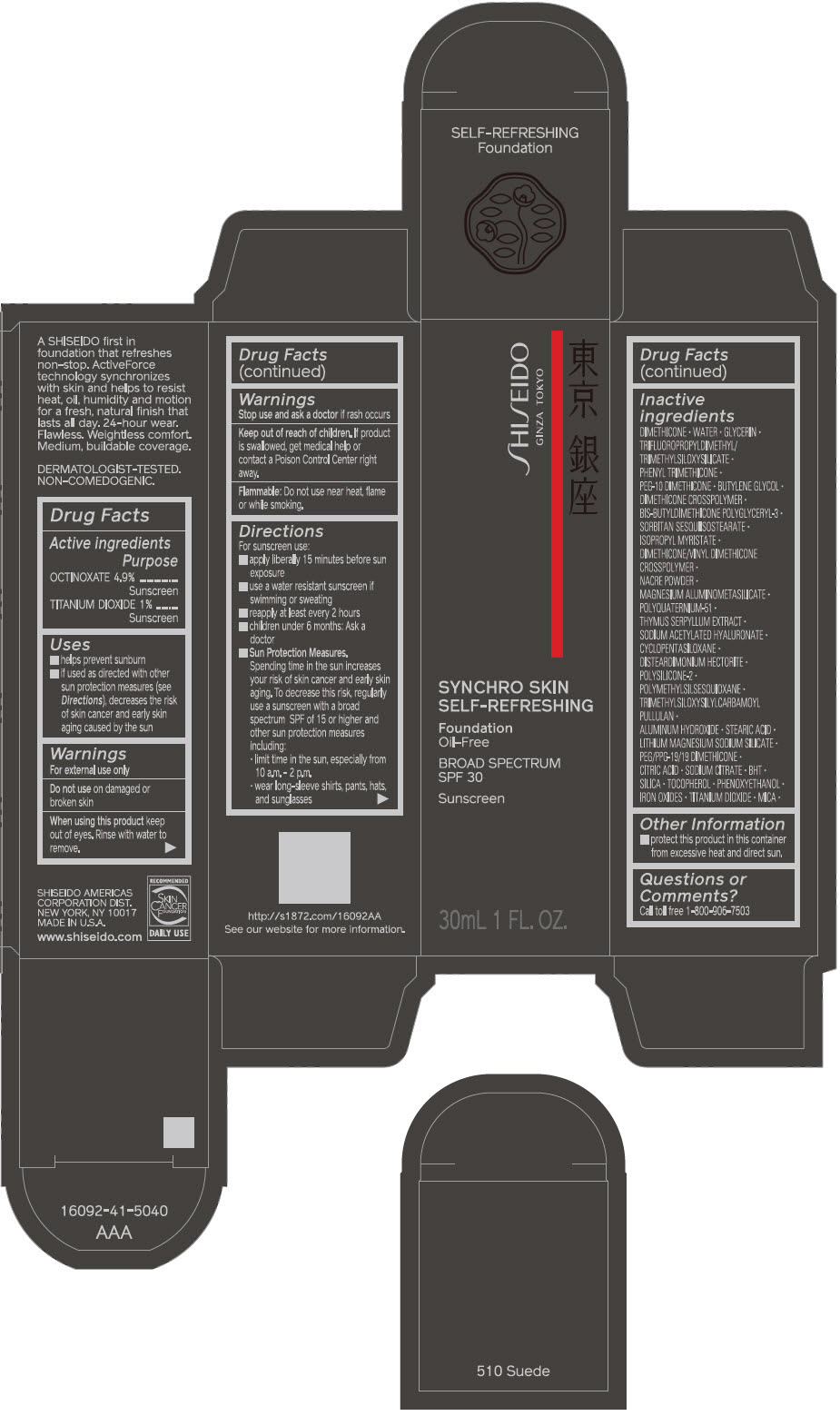
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 510 Suede
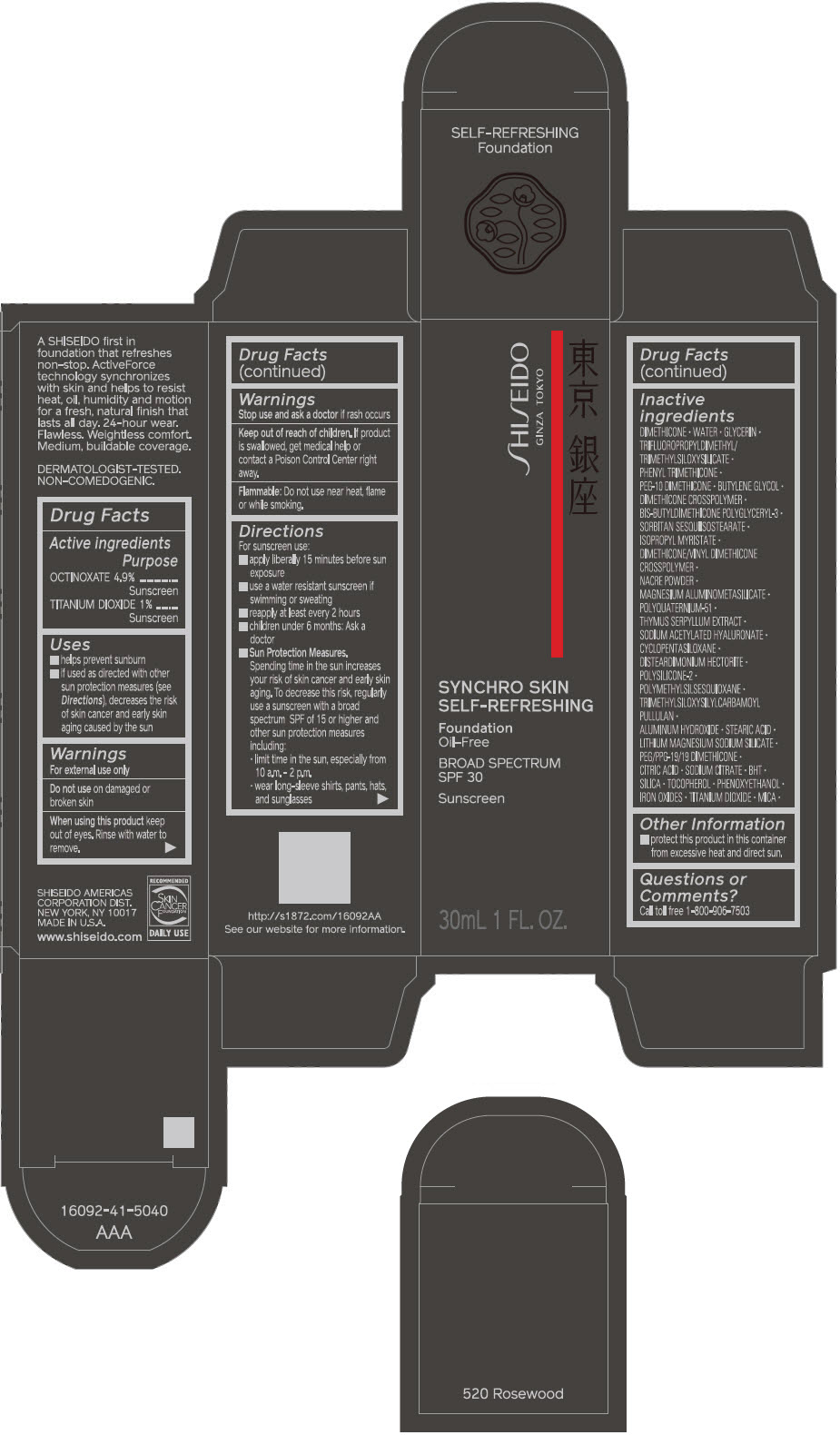
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 520 Rosewood
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 530 Henna
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 540 Mahogany
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 550 Jasper
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 560 Obsidian
-
INGREDIENTS AND APPEARANCE
SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 430 CEDAR
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-504 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-504-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 440 AMBER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-505 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-505-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 450 COPPER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-506 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-506-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 460 TOPAZ
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-507 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-507-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 510 SUEDE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-508 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-508-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 520 ROSEWOOD
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-509 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-509-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 530 HENNA
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-510 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-510-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 540 MAHOGANY
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-511-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 550 JASPER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-512-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 SHISEIDO SYNCHRO SKIN SELF-REFRESHING FOUNDATION 560 OBSIDIAN
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-513 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.69 g in 30 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.345 g in 30 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIISOSTEARATE (UNII: VU97D01BF9) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILODRATE (UNII: 9T3UU8T0QK) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) THYMUS SERPYLLUM (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) LAPONITE (UNII: D703131383) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-513-10 1 in 1 CARTON 09/01/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 09/01/2019 Labeler - SHISEIDO AMERICAS CORPORATION (193691821) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICAS INC. 782677132 MANUFACTURE(58411-504, 58411-505, 58411-506, 58411-507, 58411-508, 58411-509, 58411-510, 58411-511, 58411-512, 58411-513) , ANALYSIS(58411-504, 58411-505, 58411-506, 58411-507, 58411-508, 58411-509, 58411-510, 58411-511, 58411-512, 58411-513)