Label: NIGHTTIME CONTROL- benzoyl peroxide lotion
- NDC Code(s): 76458-333-02
- Packager: Circadia by Dr Pugliese, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
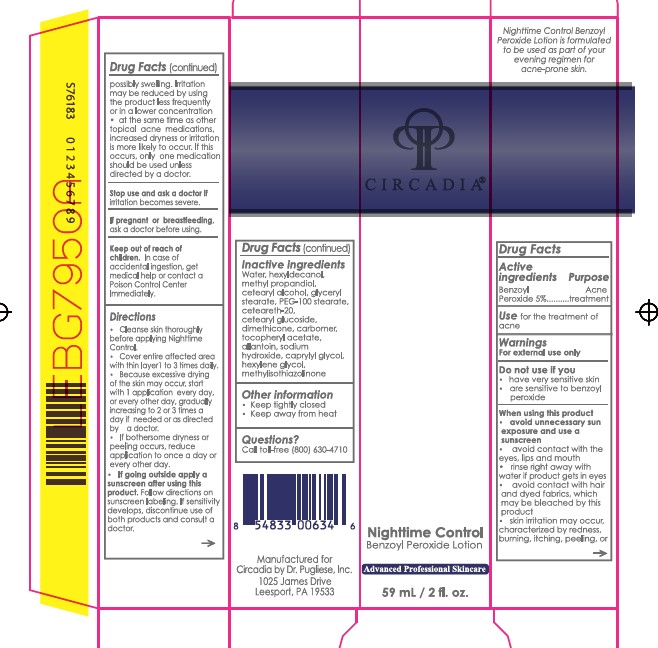
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use if you
When using this product
- have very sensitive skin
- are sensitive to benzoyl peroxide
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration
- at the same time as other topical acne medications, increased dryness or irritation is more likely to occur. If this occurs, only one medication should be used unless directed by a doctor.
- Stop use and ask a doctor
- Keep Out of Reach of Children
-
Directions
- Cleanse skin thoroughly before applying Nighttime Control.
- Cover entire affected area with a thin layer 1 to 3 times daily.
- Because excessive drying of the skin may occur, start with 1 application every day, or every other day, gradually increasing to 2 or 3 times a day if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Follow directions on sunscreen labeling. If sensitivity develops, discontinue use of both products and consult a doctor.
If going outside apply a sunscreen after using this product.
- Inactive ingredients
- Other Information
- Questions?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NIGHTTIME CONTROL
benzoyl peroxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76458-333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) WATER (UNII: 059QF0KO0R) HEXYLDECANOL (UNII: 151Z7P1317) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) PEG-100 STEARATE (UNII: YD01N1999R) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) DIMETHICONE (UNII: 92RU3N3Y1O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALLANTOIN (UNII: 344S277G0Z) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76458-333-02 1 in 1 CARTON 07/15/2012 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/15/2012 Labeler - Circadia by Dr Pugliese, Inc. (013694423) Establishment Name Address ID/FEI Business Operations Circadia by Dr Pugliese, Inc. 013694423 manufacture(76458-333)