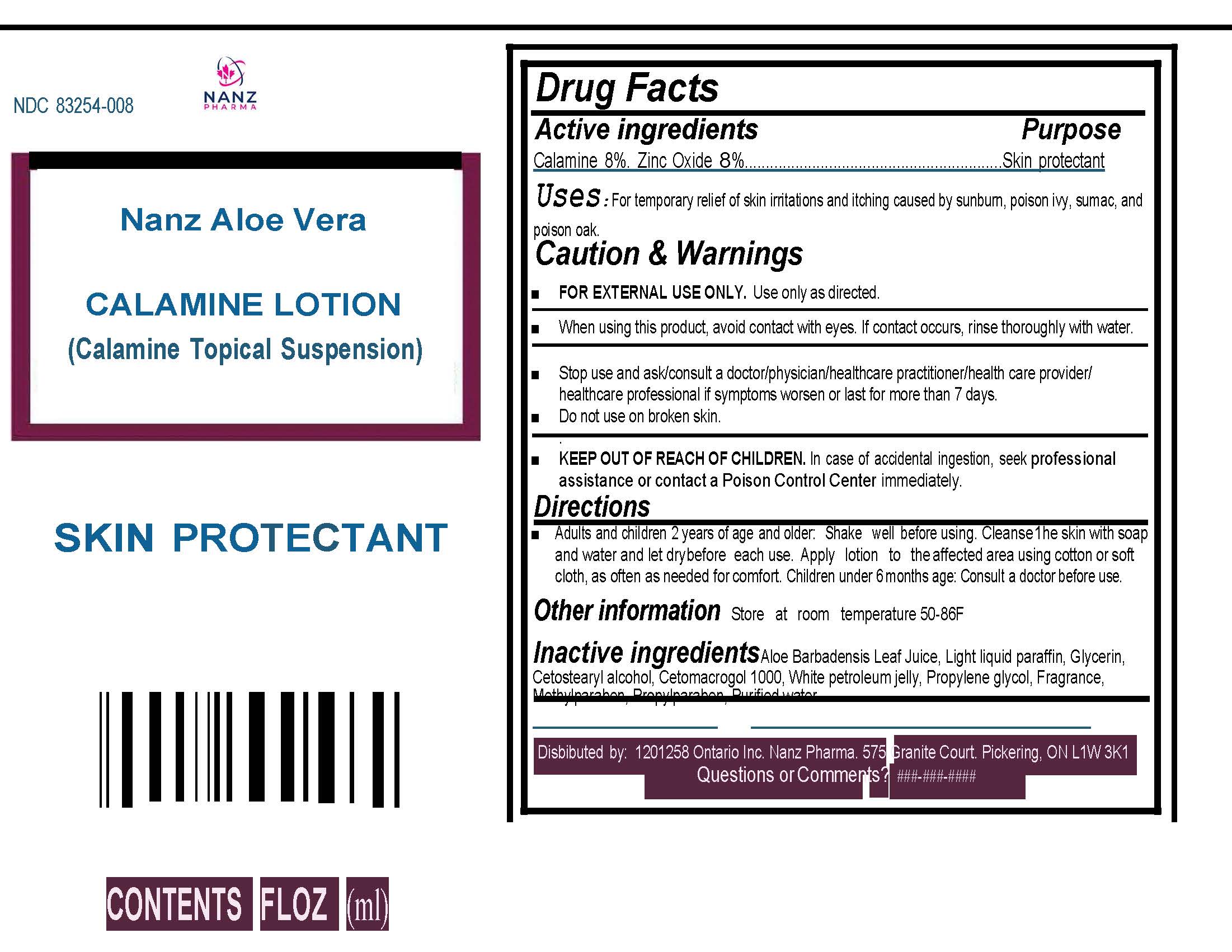
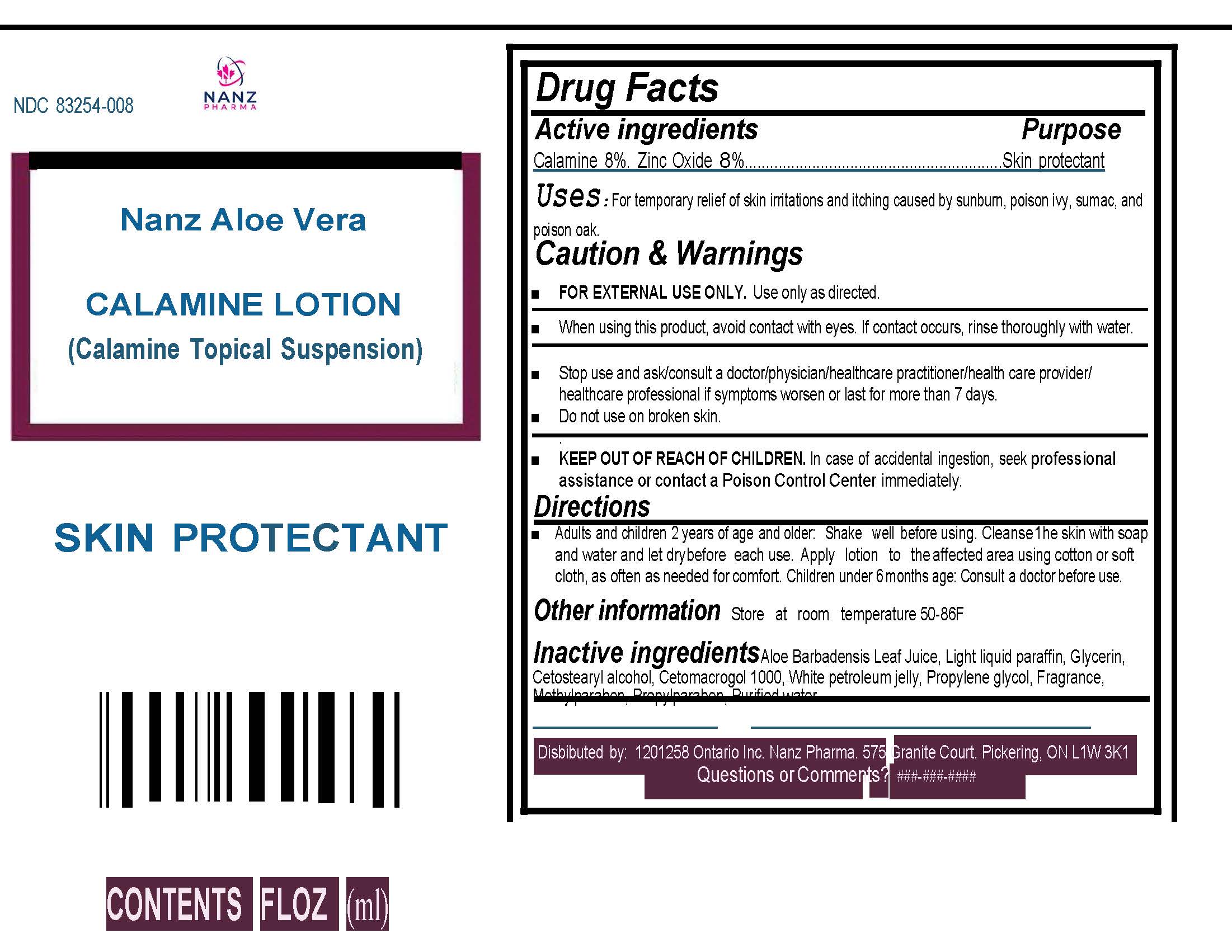
Label: ALOE VERA CALAMINE- nanz aloe vera calamine lotion lotion
-
NDC Code(s):
83254-008-01,
83254-008-02,
83254-008-05,
83254-008-06, view more83254-008-10, 83254-008-15, 83254-008-20, 83254-008-22, 83254-008-25, 83254-008-50
- Packager: 1201258 Ontario Inc O/A Nanz Pharma
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 5, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose:
- Uses:
-
Caution & Warnings:
For external use only.Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away. Stop use and ask/consult a doctor/physician/health care practitioner/health care provider/health care professional if symptoms worsen or last for more than 7 days.When using this product, avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Do not use on broken skin. - KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredients:
- Storage
- Label
-
INGREDIENTS AND APPEARANCE
ALOE VERA CALAMINE
nanz aloe vera calamine lotion lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83254-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 8 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 8 g in 100 mL Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) 10 g in 100 mL FRAGRANCE 13576 (UNII: 5EM498GW35) PROPYLPARABEN (UNII: Z8IX2SC1OH) WHITE PETROLATUM (UNII: B6E5W8RQJ4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETETH-20 (UNII: I835H2IHHX) ALOE VERA LEAF (UNII: ZY81Z83H0X) 10 g in 100 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83254-008-15 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 2 NDC:83254-008-02 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 3 NDC:83254-008-22 225 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 4 NDC:83254-008-20 2000 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 5 NDC:83254-008-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 6 NDC:83254-008-25 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 7 NDC:83254-008-10 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 8 NDC:83254-008-05 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 9 NDC:83254-008-50 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 10 NDC:83254-008-06 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M016 05/05/2023 Labeler - 1201258 Ontario Inc O/A Nanz Pharma (256906595) Registrant - 1201258 Ontario Inc. O/A Nanz Pharma (256906595) Establishment Name Address ID/FEI Business Operations 1201258 Ontario Inc. O/A Nanz Pharma 256906595 manufacture(83254-008) , label(83254-008) , pack(83254-008)