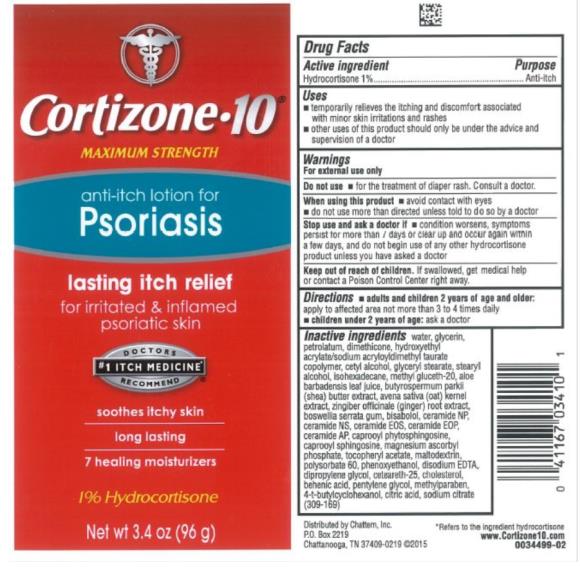
Label: CORTIZONE 10 FOR PSORIASIS- hydrocortizone lotion
- NDC Code(s): 41167-0341-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
- avoid contact with eyes
- Directions
-
Inactive ingredients
water, glycerin, petrolatum, dimethicone, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, cetyl alcohol, glyceryl stearate, stearyl alcohol, isohexadecane, methyl gluceth-20, aloe barbadensis leaf juice, butyrospermum parkii (shea) butter extract, avena sativa (oat) kernel extract, zingiber officinale (ginger) root extract, boswellia serrata gum, bisabolol, ceramide NP, ceramide NS, ceramide EOS, ceramide EOP, ceramide AP, caprooyl phytosphingosine, caprooyl sphingosine, magnesium ascorbyl phosphate, tocopheryl acetate, maltodextrin, polysorbate 60, phenoxyethanol, disodium EDTA, dipropylene glycol, ceteareth-25, cholesterol, behenic acid, pentylene glycol, methylparaben, 4-t-butylcyclohexanol, citric acid, sodium citrate
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CORTIZONE 10 FOR PSORIASIS
hydrocortizone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) ISOHEXADECANE (UNII: 918X1OUF1E) METHYL GLUCETH-20 (UNII: J3QD0LD11P) ALOE VERA LEAF (UNII: ZY81Z83H0X) SHEA BUTTER (UNII: K49155WL9Y) OAT (UNII: Z6J799EAJK) GINGER (UNII: C5529G5JPQ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) BISABOLOL OXIDE B, (-)- (UNII: S32G48306Y) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 2 (UNII: C04977SRJ5) CERAMIDE EOS (UNII: CR0J8RN66K) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 6 II (UNII: F1X8L2B00J) N-HEXANOYLSPHINGOSINE (UNII: 038753E78J) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) POLYSORBATE 60 (UNII: CAL22UVI4M) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) DIPROPYLENE GLYCOL (UNII: E107L85C40) CETEARETH-25 (UNII: 8FA93U5T67) CHOLESTEROL (UNII: 97C5T2UQ7J) BEHENIC ACID (UNII: H390488X0A) PENTYLENE GLYCOL (UNII: 50C1307PZG) METHYLPARABEN (UNII: A2I8C7HI9T) 4-TERT-BUTYLCYCLOHEXANOL (UNII: K0H1405S9C) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0341-0 96 g in 1 TUBE; Type 0: Not a Combination Product 01/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/15/2016 Labeler - Chattem, Inc. (003336013)