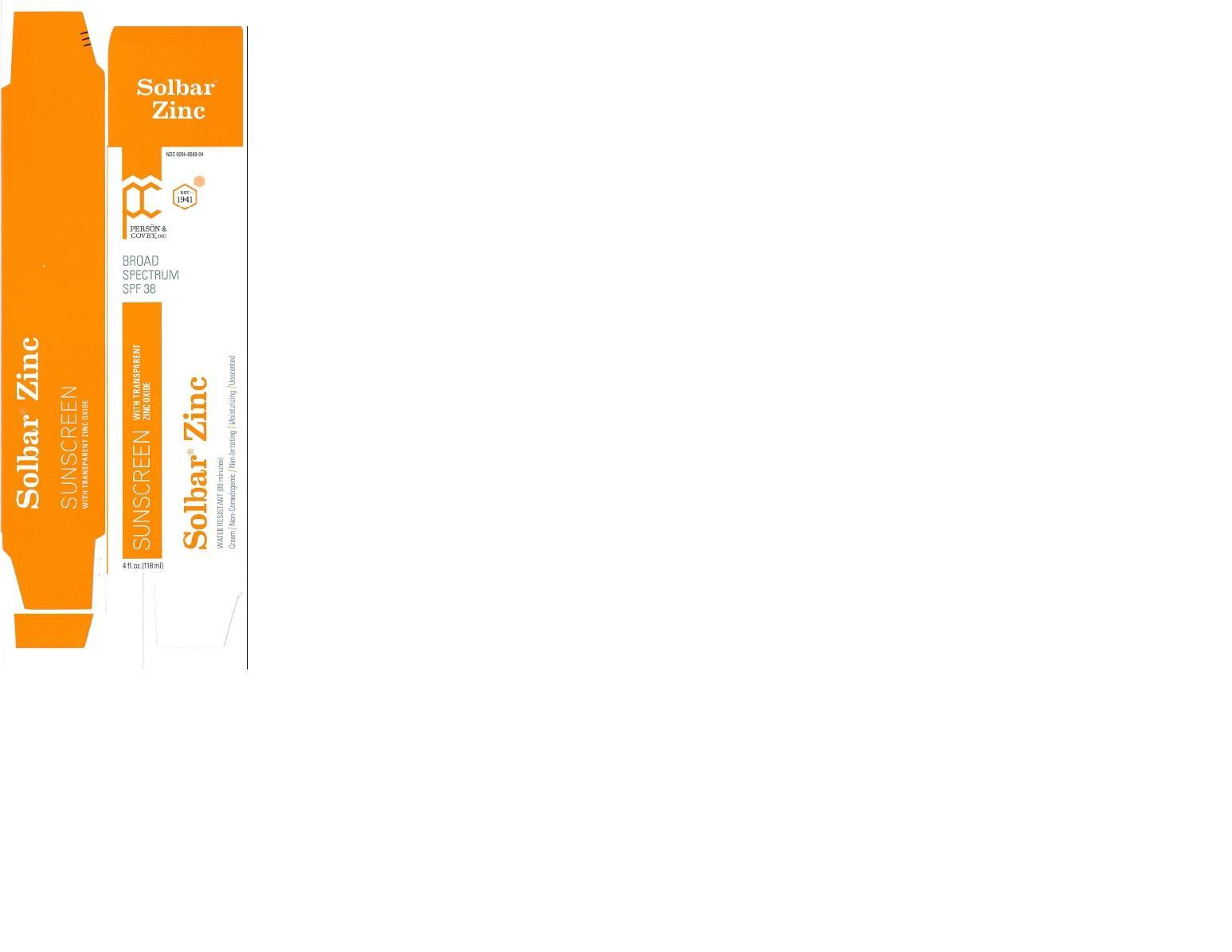
Label: SOLBAR ZINC SPF38 cream
- NDC Code(s): 0096-0688-04
- Packager: Person and Covey
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Indications and use
- Purpose
- Keep out of the reach of children
- Dosage and Administration
- Warnings
- OTC - ACTIVE INGREDIENT SECTION
- INACTIVE INGREDIENT SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOLBAR ZINC SPF38
solbar zinc spf38 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0096-0688 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.1015 g in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.077 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.077 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOBUTYL STEARATE (UNII: V8DPR6HNX3) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) EICOSYL POVIDONE (2 EICOSYL BRANCHES/REPEAT) (UNII: XQQ9MKE2BJ) GLYCERYL DILAURATE (UNII: MFL3ZIE8SK) CETYL ALCOHOL (UNII: 936JST6JCN) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) BENZYL ALCOHOL (UNII: LKG8494WBH) CYCLOMETHICONE (UNII: NMQ347994Z) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE SODIUM (UNII: MP1J8420LU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0096-0688-04 115 g in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/1996 Labeler - Person and Covey (008482473) Establishment Name Address ID/FEI Business Operations Person and Covey 008482473 manufacture(0096-0688)