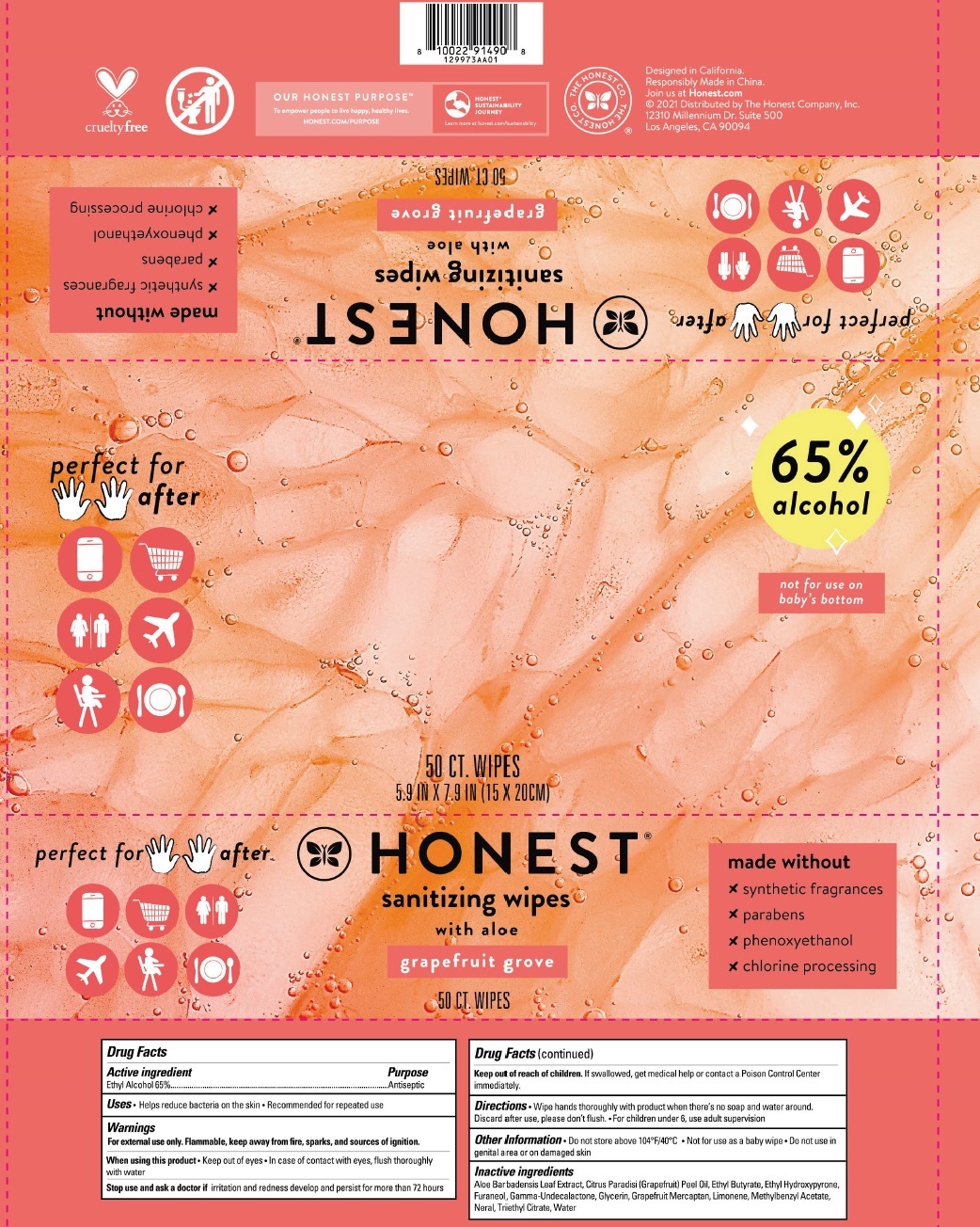
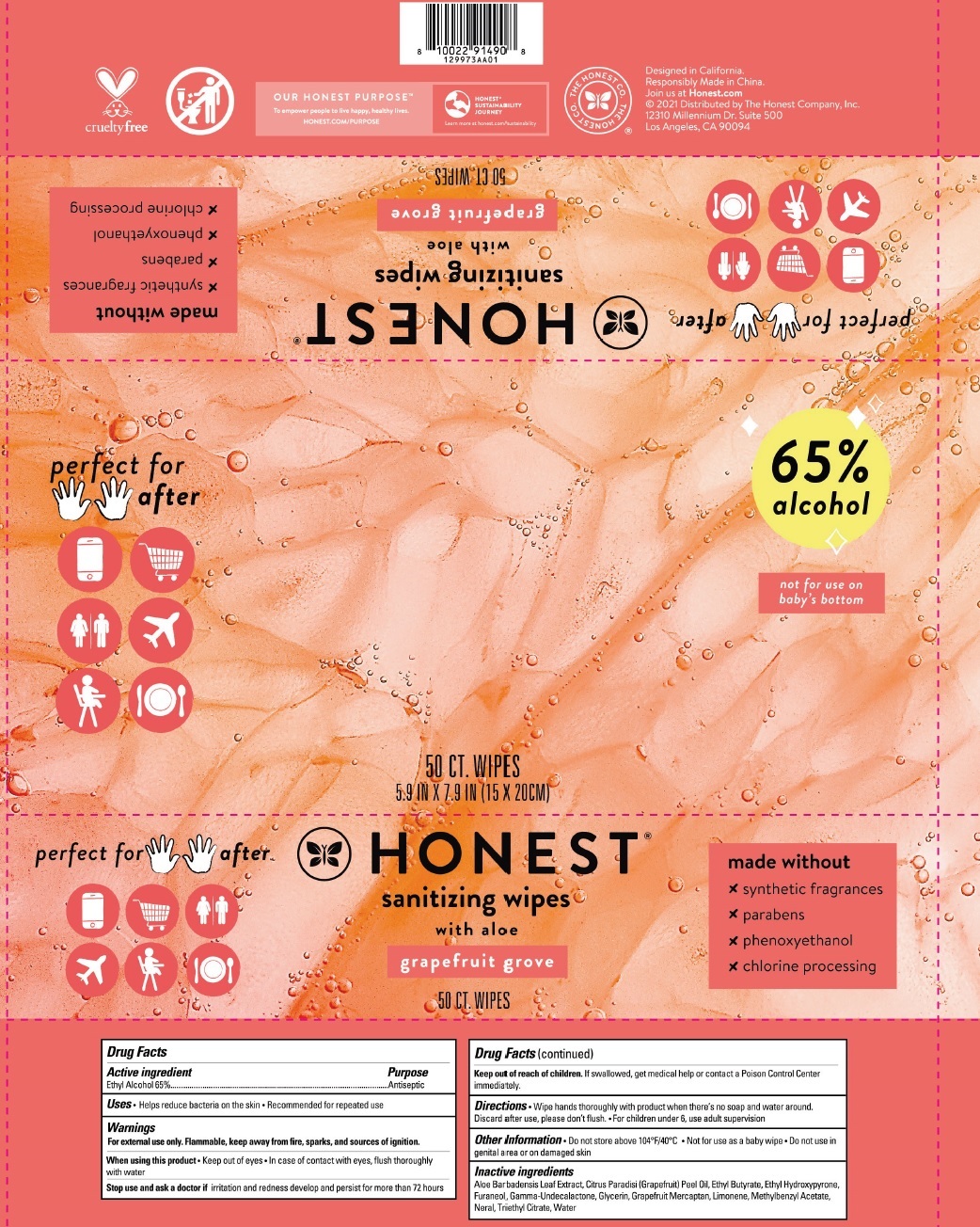
Label: SANITIZING WIPES WITH ALOE GRAPEFRUIT GROVE- alcohol cloth
- NDC Code(s): 69366-317-24, 69366-317-25, 69366-317-26
- Packager: The Honest Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Package Labeling:69366-317-25
- Package Labeling:69366-317-26
- Package Labeling:69366-317-24
-
INGREDIENTS AND APPEARANCE
SANITIZING WIPES WITH ALOE GRAPEFRUIT GROVE
alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69366-317 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GRAPEFRUIT OIL (UNII: YR377U58W9) ETHYL BUTYRATE (UNII: UFD2LZ005D) ETHYL MALTOL (UNII: L6Q8K29L05) DIMETHYLHYDROXY FURANONE (UNII: 20PI8YZP7A) .GAMMA.-UNDECALACTONE (UNII: QB1T0AG2YL) GLYCERIN (UNII: PDC6A3C0OX) 1-P-MENTHENE-8-THIOL (UNII: 7AT54D0N8R) LIMONENE, (+)- (UNII: GFD7C86Q1W) METHYLBENZYL ACETATE (UNII: P4RVP859F5) NERAL (UNII: 8M466BQL1X) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69366-317-26 15 in 1 POUCH 05/01/2022 1 4.32 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:69366-317-24 50 in 1 POUCH 05/01/2022 06/30/2024 2 4.32 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:69366-317-25 3 in 1 PACKAGE 05/01/2022 06/30/2024 3 50 in 1 POUCH 3 4.32 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/01/2022 Labeler - The Honest Company (969962757)