Label: ELELYSO- taliglucerase alfa injection, powder, lyophilized, for solution
- NDC Code(s): 0069-0106-01
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ELELYSO safely and effectively. See full prescribing information for ELELYSO.
ELELYSO® (taliglucerase alfa) for injection, for intravenous use
Initial U.S. Approval: 2012WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
See full prescribing information for complete boxed warning.
- •
- Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. (5.1)
- •
- Initiate ELELYSO in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. (5.1)
- •
- If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue ELELYSO and immediately initiate appropriate medical treatment, including use of epinephrine. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ELELYSO is a hydrolytic lysosomal glucocerebroside-specific enzyme indicated for the treatment of patients 4 years and older with a confirmed diagnosis of Type 1 Gaucher disease (1).
DOSAGE AND ADMINISTRATION
Recommendations Prior to ELELYSO Treatment (2.1):
- •
- Administration of ELELYSO should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including anaphylaxis.
Recommended Dosage in Patients 4 Years and Older (2.2):
- •
- Treatment-naïve: 60 units/kg administered every other week as a 60- to 120-minute intravenous infusion.
- •
- Patients switching from imiglucerase: Initiate ELELYSO intravenous treatment (60- to 120-minute infusion) with the same units/kg imiglucerase dosage and subsequently administer ELELYSO every other week. Dosage adjustments can be based on achievement and maintenance of each patient’s therapeutic goals.
Preparation and Administration (2.3, 2.4, 2.5):
- •
- Reconstitute, dilute and administer under the supervision of a healthcare professional.
- •
- See Full Prescribing Information for complete instructions.
DOSAGE FORMS AND STRENGTHS
For injection: 200 units lyophilized powder in a single-dose vial for reconstitution (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
See boxed warning (5.1)
ADVERSE REACTIONS
The most common adverse reactions are:
- •
- Treatment-Naïve Adults (≥5%): headache, arthralgia, fatigue, nausea, dizziness, abdominal pain, pruritus, flushing, vomiting, urticaria (6.1).
- •
- Patients who Switched from Imiglucerase, after 9 Months on Treatment (≥10%): arthralgia, headache, pain in extremity (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommendations Prior to ELELYSO Treatment
2.2 Recommended Dosage in Patients 4 Years and Older
2.3 Preparation Instructions
2.4 Storage and Handling of the Reconstituted and Diluted Solution
2.5 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions Including Anaphylaxis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials of ELELYSO as Initial Therapy
14.2 Clinical Trial in Patients Switching from Imiglucerase Treatment to ELELYSO
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life‑threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate ELELYSO in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue ELELYSO and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life‑threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommendations Prior to ELELYSO Treatment
Administration of ELELYSO should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including anaphylaxis [see Warnings and Precautions (5.1)].
Initiate ELELYSO in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment [see Warnings and Precautions (5.1)].
To reduce the risk of hypersensitivity reactions, consider pretreatment with antihistamines and/or corticosteroids [see Warnings and Precautions (5.1)].
2.2 Recommended Dosage in Patients 4 Years and Older
Treatment-naïve Patients 4 Years of Age and Older
The recommended dosage of ELELYSO is 60 units/kg (based on actual body weight) administered every other week as a 60- to 120-minute intravenous infusion.
Patients 4 Years of Age and Older Switching from Imiglucerase
If it is acceptable to switch from a stable imiglucerase dosage to ELELYSO, initiate ELELYSO intravenous treatment (60- to 120-minute infusion) with the same units/kg imiglucerase dosage and subsequently administer ELELYSO every other week. Dosage adjustments can be made based on achievement and maintenance of each patient's therapeutic goals.
2.3 Preparation Instructions
ELELYSO should be reconstituted, diluted, and administered under the supervision of a healthcare professional.
Prepare ELELYSO according to the following steps using aseptic technique:
- a.
- Determine the number of vials to be reconstituted based on the patient's weight in kg and the recommended dose [see Dosage and Administration (2.2)]. Round the number of vials up to the next whole number.
- b.
- Remove the required number of vials from the refrigerator. Do not leave these vials at room temperature longer than 24 hours prior to reconstitution. Do not heat or microwave these vials.
- c.
- Reconstitute each vial of ELELYSO with 5.1 mL of Sterile Water for Injection, USP to yield a reconstituted product with a concentration of 40 units/mL and an extractable volume of 5 mL.
- (1)
- Upon reconstitution, mix vials gently. DO NOT SHAKE.
- (2)
- Prior to further dilution, visually inspect the reconstituted solution in the vials for particulate matter and discoloration. The solution should be clear and colorless. Discard if particulate matter is present or the solution is discolored.
- d.
- Withdraw the calculated dose of drug from the appropriate number of vials and dilute with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 to 200 mL. Discard any unused reconstituted solution.
- (1)
- For pediatric patients 4 years of age and older, use a final volume of 100 to 120 mL.
- (2)
- For adult patients, may use a final volume of 130 to 150 mL. However, if the volume of reconstituted product alone is equal to or greater than 130 to 150 mL, then the final volume should not exceed 200 mL.
- e.
- Mix the diluted solution gently. DO NOT SHAKE. Since this is a protein solution, slight flocculation (described as translucent fibers) occurs occasionally after dilution.
- f.
- Discard any unused diluted solution.
2.4 Storage and Handling of the Reconstituted and Diluted Solution
- •
- If the reconstituted ELELYSO vial is not used immediately, refrigerate at 2 °C to 8 °C (36 °F to 46 °F) for up to 24 hours (under protection from light) or store at controlled room temperature at 20 °C to 25 °C (68 °F to 77 °F) for up to 4 hours (without protection from light).
- •
- If the diluted solution is not administered immediately, refrigerate at 2 °C to 8 °C (36 °F to 46 °F) for up to 24 hours (under protection from light).
- •
- The total storage time for the reconstituted and diluted solution should not exceed 24 hours. Discard the unused reconstituted or diluted solution after 24 hours from the start of preparation.
- •
- Do not freeze.
2.5 Administration Instructions
After reconstitution and dilution, administer via intravenous infusion over a minimum of 60 minutes and with an in‑line low protein-binding 0.2 micron filter.
- •
- For pediatric patients who weigh (based on actual body weight):
- o
- Less than 30 kg use an infusion rate of 1 mL/minute.
- o
- Greater than or equal to 30 kg, use an initial infusion rate of 1 mL/minute. After tolerability to ELELYSO is established, may increase the infusion rate to a maximum of 2 mL/minute.
- •
- For adult patients, use an initial infusion rate of 1.2 mL/minute. After tolerability to ELELYSO is established, may increase the infusion rate to a maximum of 2.2 mL/minute.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions Including Anaphylaxis
Life‑threatening hypersensitivity reactions, including anaphylaxis, have occurred in patients treated with enzyme replacement therapies, including ELELYSO. In clinical trials, (patients were not routinely pretreated with antihistamines and/or corticosteroids prior to ELELYSO infusions during the clinical trials):
- •
- 2 of 72 (3%) ELELYSO-treated patients experienced signs and symptoms consistent with anaphylaxis including urticaria, hypotension, flushing, wheezing, chest tightness, nausea, vomiting, and dizziness. These reactions occurred during ELELYSO infusion.
- •
- 21 of 72 (29%) ELELYSO-treated patients experienced hypersensitivity reactions, including the 2 ELELYSO‑treated patients who experienced signs and symptoms consistent with anaphylaxis. Signs and symptoms of these hypersensitivity reactions included pruritus, angioedema, flushing, erythema, rash, nausea, vomiting, cough, chest tightness, and throat irritation. These reactions occurred during ELELYSO infusion and up to 3 hours after the start of infusion [see Adverse Reactions (6.1)].
Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Administration of ELELYSO should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions including anaphylaxis. Initiate ELELYSO in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. Observe patients closely during and after the infusion.
ELELYSO-treated patients who developed anti-taliglucerase alfa antibodies (referred to as anti-drug antibodies (ADA)) generally had a greater frequency of hypersensitivity reactions compared to those who did not develop ADA [see Adverse Reactions (6.1)]. Closely monitor for hypersensitivity reactions in patients who develop ADA.
Management of hypersensitivity reactions should be based on the severity of the reaction and includes slowing or temporary interruption of the infusion and/or administration of antihistamines, antipyretics, and/or corticosteroids for mild reactions. To reduce the risk of hypersensitivity reactions, consider pretreatment with antihistamines and/or corticosteroids. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue ELELYSO and immediately initiate appropriate medical treatment, including use of epinephrine.
Inform patients of the symptoms of life‑threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur.
Consider the risks and benefits of re-administering ELELYSO in patients who have experienced a severe hypersensitivity reaction associated with ELELYSO. Caution should be exercised upon rechallenge [see Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials of ELELYSO as Initial Therapy
- •
- Clinical Trial in Adult Patients
- The safety of ELELYSO at dosages of either 30 units/kg (n=16) (50% of the recommended dosage) [see Dosage and Administration (2.1)] or 60 units/kg (n=16) administered intravenously every other week was assessed in 32 adult treatment-naïve patients (aged 19 to 74 years) with Type 1 Gaucher disease in a 9-month double-blind, randomized clinical trial (Trial 1) [see Clinical Studies (14)]. Table 1 presents the adverse reactions that occurred in these ELELYSO-treated patients.
Table 1: Adverse Reactions in ≥5% of Treatment-Naïve Adult Patients Treated with ELELYSO Preferred Term
Treatment-Naïve Adults (N=32)
n (%)Headache
6 (19)
Arthralgia
4 (13)
Fatigue
3 (9)
Nausea
3 (9)
Dizziness
3 (9)
Abdominal pain
2 (6)
Pruritus
2 (6)
Flushing
2 (6)
Vomiting
2 (6)
Urticaria
2 (6)
- •
- Clinical Trial in Pediatric Patients 16 Years of Age and Younger
- The safety of ELELYSO at dosages of either 30 units/kg (n=4) (50% of the recommended dosage) [see Dosage and Administration (2.1)] or 60 units/kg (n=5) administered intravenously every other week was assessed in 9 pediatric treatment-naïve patients (aged 2 to 13 years) with Type 1 Gaucher disease in a 12-month randomized clinical trial (Trial 2) [see Clinical Studies (14)].
The most common adverse reaction (≥10%) was vomiting, which occurred in 4 of 9 patients. Two patients developed hypersensitivity reactions; one patient experienced severe vomiting and gastrointestinal inflammation, and 1 experienced mild throat irritation and chest discomfort. Both patients responded to treatment with antihistamines and continued ELELYSO treatment.
Adverse Reactions in a Clinical Trial in Patients Who Switched from Imiglucerase to ELELYSO
The safety of ELELYSO was assessed in 31 patients (26 adult and 5 pediatric patients), ages 6 to 66 years old, with Type 1 Gaucher disease who had previously been receiving imiglucerase treatment for a minimum of 2 years (Trial 3). ELELYSO was administered intravenously every other week for 9 months at the same number of units as each patient's previous imiglucerase dose. Table 2 presents the adverse reactions in these ELELYSO-treated patients.
Table 2: Adverse Reactions in ≥10% of ELELYSO-Treated Patients Who Switched from Imiglucerase to ELELYSO (after 9 months on treatment) Preferred Term
Adult and Pediatric Patients Switched from Imiglucerase
(N=31)
n (%)Arthralgia
4 (13)
Headache
4 (13)
Pain in extremity
3 (10)
Immunogenicity: Anti-Drug Antibody-Associated Adverse Reactions
Trials 1, 2, and 3 evaluated ELELYSO enzyme replacement therapy (ERT)-naïve and ERT-experienced adult and pediatric patients with Gaucher disease [see Clinical Studies (14.1, 14.2)]. In patients with Type 1 Gaucher disease, hypersensitivity reactions occurred in 36% (9/25) of ELELYSO-treated patients who developed ADA during the treatment period and in 15% (6/41) of ELELYSO-treated patients who did not develop ADA during the treatment period [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.6)]. Of the 9 ELELYSO-treated patients who tested positive for ADA and who developed hypersensitivity reactions, 2 patients had anaphylaxis and 1 additional patient discontinued ELELYSO due to hypersensitivity reactions.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ELELYSO. Because these reactions include those reported voluntarily from a population of uncertain size in addition to those from postmarketing studies, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- •
- Gastrointestinal disorders: Vomiting, diarrhea
- •
- General disorders and administration site conditions: Fatigue
- •
- Immune system disorders: Anaphylaxis [see Warnings and Precautions (5.1)], Type III immune-mediated fixed drug eruption
- •
- Musculoskeletal and connective tissue disorders: Back pain
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on ELELYSO use in pregnant women are not sufficient to inform a drug-associated risk. However, there are clinical considerations (see Clinical Considerations). In animal reproduction studies when pregnant rats and rabbits were administered taliglucerase alfa at intravenous doses up to 5 times the recommended human dose (RHD), there was no evidence of embryo-fetal toxicity (see Data). The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Disease-Associated Maternal and/or Embryo/Fetal Risk
Women with Type 1 Gaucher disease have an increased risk of spontaneous abortion if disease symptoms are not treated and controlled pre-conception and during a pregnancy. Pregnancy may exacerbate existing Type 1 Gaucher disease symptoms or result in new disease manifestations. Type 1 Gaucher disease manifestations may lead to adverse pregnancy outcomes, including hepatosplenomegaly which can interfere with the normal growth of a fetus and thrombocytopenia which can lead to increased bleeding and possible postpartum hemorrhage requiring transfusion.
Animal Data
Reproduction studies have been performed with taliglucerase alfa administered during the period of organogenesis in rats and rabbits. In rats, intravenous doses up to 55 mg/kg/day (about 5 times the RHD of 60 units/kg based on the body surface area) did not cause any adverse effects on embryo-fetal development. In rabbits, intravenous doses up to 27.8 mg/kg/day (about 5 times the RHD of 60 units/kg based on the body surface area) did not show any embryo-fetal toxicity.
8.2 Lactation
Risk Summary
There are no data on the presence of taliglucerase alfa in human milk, the effects on the breast fed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ELELYSO and any potential adverse effects on the breastfed child from ELELYSO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ELELYSO for the treatment of pediatric patients 4 years of age and older with a confirmed diagnosis of Type 1 Gaucher disease has been established. The use of ELELYSO for this indication is supported by evidence of effectiveness from adequate and well-controlled trials of ELELYSO in adults, with additional pharmacodynamic data from 5 pediatric patients and pharmacokinetic data from 9 pediatric patients who participated in clinical trials [see Clinical Studies (14.1, 14.2), Clinical Pharmacology (12.3)]. Data from 14 pediatric patients were included in the safety evaluation [see Adverse Reactions (6.1)]. The safety and effectiveness of ELELYSO has not been established in patients less than 4 years of age.
Pediatric patients experienced a higher frequency of vomiting during ELELYSO treatment (4 of 9 treatment-naïve patients) than adult patients, and this may be a symptom of hypersensitivity reaction. The frequencies of other adverse reactions were similar between pediatric and adult patients treated with ELELYSO [see Adverse Reactions (6.1)].
-
11 DESCRIPTION
Taliglucerase alfa is a hydrolytic lysosomal glucocerebroside-specific enzyme produced by recombinant DNA technology using plant cell culture (carrot). Taliglucerase alfa is a monomeric glycoprotein enzyme containing 4 N-linked glycosylation sites (kDa=60.8). Taliglucerase alfa differs from native human glucocerebrosidase by two amino acids at the N terminal and up to 7 amino acids at the C terminal. Taliglucerase alfa is a glycosylated protein with oligosaccharide chains at the glycosylation sites having terminal mannose sugars. These mannose-terminated oligosaccharide chains of taliglucerase alfa are specifically recognized by endocytic carbohydrate receptors on macrophages, the cells that accumulate lipid in Gaucher disease.
A unit is the amount of enzyme that catalyzes the hydrolysis of 1 micromole of the synthetic substrate para-nitrophenyl-β-D-glucopyranoside (pNP-Glc) per minute at 37 °C.
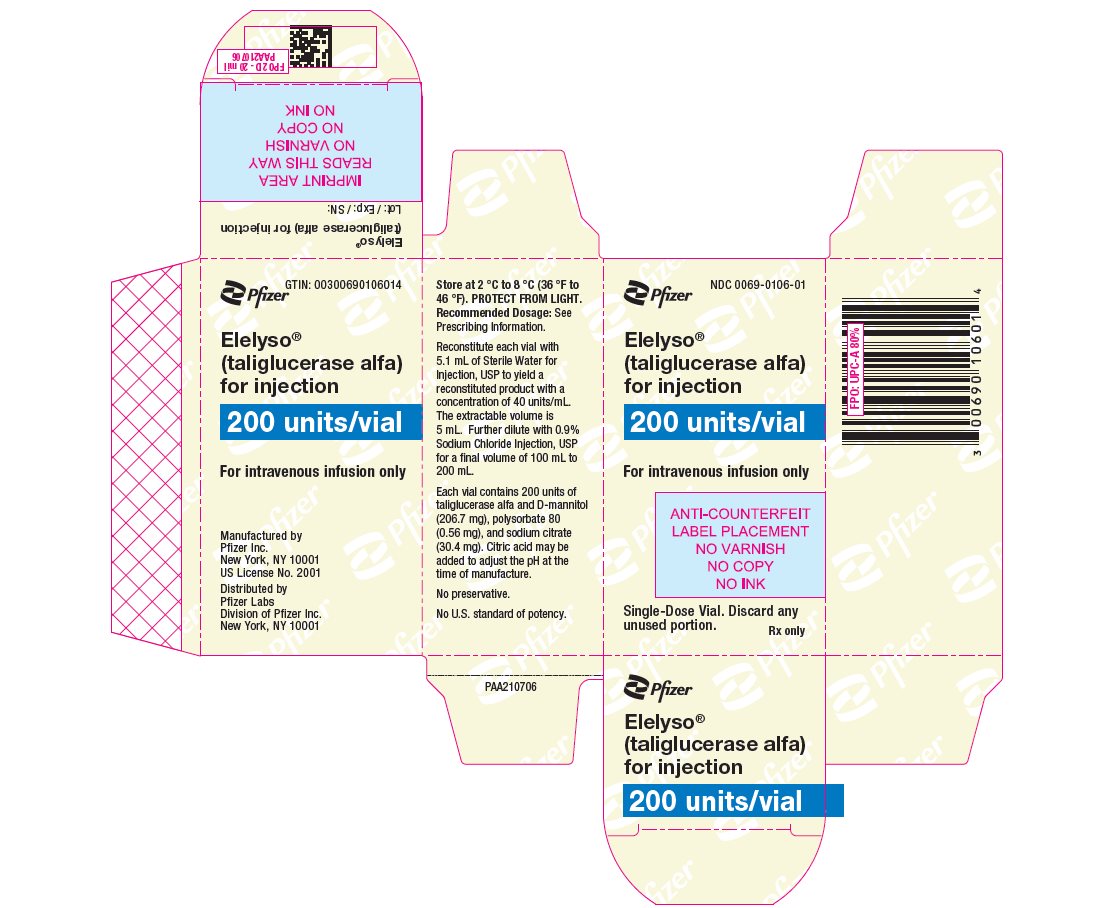
ELELYSO (taliglucerase alfa) for injection is supplied as a sterile, preservative-free, lyophilized powder for reconstitution and dilution prior to intravenous infusion. Each single-dose vial contains 200 units of taliglucerase alfa and D-mannitol (206.7 mg), polysorbate 80 (0.56 mg), and sodium citrate (30.4 mg). Citric acid may be added to adjust the pH at the time of manufacture. After reconstitution with 5.1 mL Sterile Water for Injection, USP, taliglucerase alfa concentration is 40 units/mL [see Dosage and Administration (2)]. Reconstituted solutions have a pH of approximately 6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gaucher disease is an autosomal recessive disorder caused by mutations in the human glucocerebrosidase gene, which results in a reduced activity of the lysosomal enzyme glucocerebrosidase. Glucocerebrosidase catalyzes the conversion of the sphingolipid glucocerebroside into glucose and ceramide. The enzymatic deficiency results in accumulation of substrate glucocerebroside primarily in the lysosomal compartment of macrophages, giving rise to foam cells or "Gaucher cells," which accumulate in the liver, spleen and bone marrow.
ELELYSO, an enzyme replacement therapy, is a recombinant analog of human lysosomal glucocerebrosidase that catalyzes the hydrolysis of glucocerebroside to glucose and ceramide, reducing the amount of accumulated glucocerebroside. ELELYSO uptake into cellular lysosomes is mediated by binding of ELELYSO mannose oligosaccharide chains to specific mannose receptors on the cell surface leading to internalization and subsequent transport to the lysosomes.
12.3 Pharmacokinetics
Pharmacokinetics of taliglucerase alfa were evaluated in 38 patients (29 adult and 9 pediatric patients) who received intravenous infusions of ELELYSO 30 units/kg (50% of the recommended dosage) or 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] every other week. The pharmacokinetic parameters in adult and pediatric patients are summarized in Table 3.
In adult Type 1 Gaucher disease patients treated with ELELYSO 30 units/kg (50% of the recommended dosage) or 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] (N=29) every other week as initial therapy, pharmacokinetics were determined with the first dose and at Week 38 of treatment. The pharmacokinetics of taliglucerase alfa appeared to be nonlinear with a greater than dose-proportional increase in exposure at the doses studied.
No significant accumulation or change in taliglucerase alfa pharmacokinetics over time from Weeks 1 to 38 was observed with repeated dosages of 30 units/kg (50% of the recommended dosage) or 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] every other week. Based on the limited data, there were no significant pharmacokinetic differences between male and female patients in this study.
The pharmacokinetics of taliglucerase alfa were evaluated in 9 pediatric patients 4 to 17 years of age with Type 1 Gaucher disease who were treated with ELELYSO for 10 to 27 months. Six of the 9 patients were treatment-naïve, and 3 patients were switched from imiglucerase. In both the 30 units/kg (50% of the recommended dosage) and 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] dose groups, clearance values in pediatric patients were similar to those in adult patients. AUC values in pediatric patients were lower than AUC values in adult patients, due to weight-based dosing of taliglucerase alfa and lower body weights in pediatric patients.
Table 3: Taliglucerase Alfa Pharmacokinetic Parameters after Repeated Dosing in Adult and Pediatric Patients with Type 1 Gaucher Disease Pediatric Patients (N=9)
Median (Range)Adult Patients at Week 38 (N=29)
Median (Range)30 units/kg*
n = 560 units/kg
n = 430 units/kg*
n = 1460 units/kg
n = 15Age (years)
15 (10, 17)
11 (4, 16)
35 (19, 74)
33 (19, 58)
Weight (kg)
44.3 (22.8, 71.0)
28.6 (16.5, 50.4)
72.5 (51.5, 99.5)
73.5 (58.5, 87.0)†
AUC0–∞ (ng*h/mL)‡
1416 (535, 1969)
2984 (1606, 4273)
2007 (1007, 10092)
6459 (2548, 21020)†
T1/2 (min)
37.1 (22.5, 56.8)
32.5 (18.0, 42.9)
18.9 (9.20, 57.9)
28.7 (11.3, 104)†
CL (L/h)
30.5 (17.4, 37.8)
15.8 (11.7, 24.9)
30.5 (6.79, 68.0)
18.5 (6.20, 37.9)†
Vss (L)
14.9 (10.1, 35.6)
8.80 (3.75, 21.4)
11.7 (2.3, 22.7)
10.7 (1.4, 18.5)†
12.6 Immunogenicity
The observed incidence of ADA (including neutralizing antibodies [Nab]) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of ELELYSO or of other taliglucerase alfa products.
Anti-Drug Antibodies
In Trials 1, 2, and 3, a greater portion of ELELYSO-treated patients who developed ADA had hypersensitivity reactions compared to those who did not develop ADA [see Adverse Reactions (6.1) and Warnings and Precautions (5.1)].
In Trial 1 (treatment-naïve adults with Gaucher disease) [see Clinical Studies (14.1)], 17 (53%) of 32 ELELYSO-treated patients developed ADA. Additionally 2 (6%) patients tested positive for ADA at baseline prior to ELELYSO treatment.
In Trial 2 (treatment-naïve pediatric patients with Gaucher disease) [see Clinical Studies (14.1)], 2 (22%) of 9 Type 1 Gaucher disease ELELYSO-treated patients developed ADA. Additionally, 1 patient was ADA-positive prior to initiation of ELELYSO.
In Trial 3 (switched from imiglucerase to ELELYSO), of 31 ELELYSO-treated patients (26 adult and 5 pediatric patients) [see Clinical Studies (14.2)], 6 adults (23% of adult patients) developed ADA and no pediatric patient developed ADA. Additionally, 3 (10%) patients were ADA positive prior to initiation of ELELYSO.
There is insufficient information to characterize the ADA response to ELELYSO and the effects of ADA on pharmacokinetics, pharmacodynamics, or effectiveness of taliglucerase alfa products.
Neutralizing Antibodies
In Trials 1, 2 and 3 with a total number of 72 patients, 30 of the 31 ELELYSO-treated adult and pediatric patients who developed ADA or tested positive for ADA at baseline were evaluated for neutralizing activity of the ADA in the mannose receptor binding and enzyme activity assays.
- o
- Nineteen (63%) of the 30 patients had neutralizing antibodies (Nab) capable of inhibiting in vitro mannose receptor binding of ELELYSO.
- o
- Eight (42%) of these 19 patients had Nab capable of inhibiting the in vitro enzymatic activity of ELELYSO.
Although the effectiveness was numerically lower (less spleen and liver volume reduction) in ELELYSO-treated patients who developed Nab compared to those that did not develop Nab, the data were not sufficient to fully assess whether the observed Nab reduces effectiveness.
Other Antibodies
Nine (29%) of the 31 ELELYSO-treated adult and pediatric patients who developed ADA during treatment or tested positive for ADA at baseline also developed antibodies against plant-specific glycans in ELELYSO.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with taliglucerase alfa. In a male and female fertility study in rats, taliglucerase alfa did not cause any significant adverse effect on male or female fertility parameters up to a maximum dose of 55 mg/kg/day (about 5 times the recommended human dose of 60 units/kg based on the body surface area).
-
14 CLINICAL STUDIES
14.1 Clinical Trials of ELELYSO as Initial Therapy
Clinical Trial in Adult Patients
The safety and efficacy of ELELYSO in the treatment of adult patients with Type 1 Gaucher disease was assessed in a 9-month, multi-center, double-blind, randomized trial (Trial 1) in 31 adult patients with Gaucher disease-related enlarged spleens (>8 times normal) and thrombocytopenia (<120,000 /mm3). Sixteen patients had enlarged livers and ten patients had anemia at baseline. All patients were naïve to enzyme replacement therapy (ERT). Patients with severe neurological symptoms were excluded from the trial.
Patients were randomized to receive ELELYSO at an intravenous dosage of either 30 units/kg (n=15) (50% of the recommended dosage) or 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] (n=16) every other week. After 9 months, 26 of the 31 patients continued in the blinded portion of the long-term extension trial for a total treatment duration of 24 months at the same intravenous dosage every other week. Twenty three of those 26 patients continued open-label ELELYSO treatment (30 or 60 units/kg given intravenously every other week) for an additional 12 months (total duration of ELELYSO treatment was 36 months).
Baseline Demographics
In Trial 1, patients were 19 to 74 years of age (mean age 36 years), 48% were male, 97% were White and 29% and 71% were Hispanic/Latino and non Hispanic/Latino, respectively.
Efficacy Results
Table 4 shows the baseline values and mean (SD) changes in clinical parameters (spleen volume, liver volume, platelet count, and hemoglobin) after 9 months of ELELYSO treatment in Trial 1. Liver and spleen volumes were measured by MRI and are reported as percentage of body weight (%BW) and multiples of normal (MN). The observed reduction from baseline in spleen volume (the primary endpoint), was considered to be clinically meaningful in light of the natural history of untreated Gaucher disease.
Table 4: Mean (SD*) Changes in Clinical Parameters from Baseline to 9 Months in Treatment-Naïve Adults with Type 1 Gaucher Disease Treated with ELELYSO (N=31) (Trial 1) - *
- SD = standard deviation
- †
- The recommended ELELYSO dosage in treatment-naïve adult patients is 60 units/kg every other week. ELELYSO 30 units/kg every other week is not a recommended dosage. [see Dosage and Administration (2.1)]
- ‡
- %BW = percentage of body weight
- §
- MN = multiples of normal
Clinical Parameter
ELELYSO
30 units/kg† (n=15)
Mean (SD)ELELYSO
60 units/kg (n=16)
Mean (SD)Spleen Volume (%BW‡)
Baseline
3.1 (1.5)
3.3 (2.7)
Month 9
2.2 (1.3)
2.1 (1.9)
Change
-0.9 (0.4)
-1.3 (1.1)
Spleen Volume (MN§)
Baseline
15.4 (7.7)
16.7 (13.4)
Month 9
11.1 (6.3)
10.4 (9.4)
Change
-4.5 (2.1)
-6.6 (5.4)
Liver Volume (%BW)
Baseline
4.2 (0.9)
3.8 (1.0)
Month 9
3.6 (0.7)
3.1 (0.7)
Change
-0.6 (0.5)
-0.6 (0.4)
Liver Volume (MN)
Baseline
1.7 (0.4)
1.5 (0.4)
Month 9
1.4 (0.3)
1.2 (0.3)
Change
-0.2 (0.2)
-0.3 (0.2)
Platelet Count (mm3)
Baseline
75,320 (40,861)
65,038 (28,668)
Month 9
86,747 (50,989)
106,531 (53,212)
Change
11,427 (20,214)
41,494 (47,063)
Hemoglobin (g/dl)
Baseline
12.2 (1.7)
11.4 (2.6)
Month 9
14.0 (1.4)
13.6 (2.0)
Change
1.6 (1.4)
2.2 (1.4)
The following data are the changes in clinical parameters from baseline to Month 24 (including the 9-month initial period and the 15-month first long-term extension) for the 30 units/kg (n=12) (50% of the recommended dosage) and 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] (n=14) treatment groups, respectively: mean (SD) spleen volume (%BW) decreased by 1.4 (0.6) and 2.0 (2.0), in MN by 6.8 (3.0) and 10.2 (9.8); hemoglobin increased by 1.3 (1.7) g/dL and 2.4 (2.3) g/dL; liver volume (%BW) decreased by 1.1 (0.5) and 1.0 (0.7), in MN by 0.4 (0.2) and 0.4 (0.3 and platelet count increased 28,433 (31,996)/mm3 and 72,029 (68,157)/mm3. The 23 patients who continued open-label ELELYSO treatment for additional 12 months demonstrated stability in these clinical parameters.
Clinical Trial in Pediatric Patients 16 Years of Age and Younger
The safety and efficacy of ELELYSO in the treatment of pediatric patients with Type 1 Gaucher disease was assessed in a 12-month, multi-center, double-blind, randomized trial (Trial 2) in 9 treatment-naïve patients. Patients were randomized to receive ELELYSO at an intravenous dosage of either 30 units/kg (n=4) (50% of the recommended dosage) or 60 units/kg (the recommended dosage) [see Dosage and Administration (2.1)] (n=5) every other week. After 12 months, all 9 patients entered a blinded portion of the long-term extension trial (24-months of total treatment) where they continued treatment with ELELYSO at the same dosage every other week.
Baseline Demographics
In Trial 2, patients were 2 to 13 years of age (mean age 8.1 years), 67% were male, 89% were White and 44% and 56% were Hispanic/Latino and non Hispanic/Latino, respectively.
Efficacy Results
The following data in Trial 2 are the changes [median (Q1, Q3)] in clinical parameters from baseline to Month 12 for the 60 units/kg dose group (n=5): spleen volume decreased from 18.4 (14.2, 35.1) MN to 11.0 (8.3, 14.5) MN; hemoglobin increased from 11.1 (9.2, 11.3) g/dL to 11.7 (11.5, 12.9) g/dL; liver volume decreased from 2.1 (2.0, 2.3) MN to 1.6 (1.5, 1.9) MN; platelet count increased from 80,000 (79,000, 87,000)/mm3 to 131,000 (119,000, 215,000)/mm3.
The following data are the changes [median (Q1, Q3)] in clinical parameters from baseline to Month 24 (including the initial 12-month period and the 12-month long-term extension) for the 60 units/kg dose group (n=5): spleen volume decreased by 19.0 (8.3, 41.2) MN; hemoglobin increased by 2.5 (1.9, 3.0) g/dL; liver volume decreased by 0.8 (0.6, 1.1) MN; and platelet count increased by 76,000 (67,000, 100,000)/mm3.
14.2 Clinical Trial in Patients Switching from Imiglucerase Treatment to ELELYSO
The safety and efficacy of ELELYSO were assessed in 31 patients (26 adult and 5 pediatric patients) with Type 1 Gaucher disease who were switched from imiglucerase to ELELYSO (Trial 3). Trial 3 was a 9-month, multi-center, open-label, single arm study in patients who had been receiving intravenous treatment with imiglucerase at dosages ranging from 9.5 units/kg to 60 units/kg every other week for a minimum of 2 years. Patients were required to be clinically stable and have a stable biweekly dosage of imiglucerase for at least 6 months prior to enrollment. Imiglucerase therapy was stopped, and treatment with ELELYSO was administered every other week at the same number of units as each patient's previous imiglucerase dose (9.5 units/kg to 60 units/kg given intravenously every other week). If needed, adjustment of dosage was allowed during the study in order to maintain stability of clinical parameters (i.e., spleen volume, liver volume, platelet count, and hemoglobin).
- •
- Eighteen of the 26 adult patients who completed the 9-month clinical trial continued treatment with ELELYSO (9.5 units/kg to 60 units/kg given intravenously every other week) in an open-label extension trial for additional 27 months (total duration of ELELYSO treatment was 36 months).
- •
- Five of the pediatric patients who completed the 9-month trial continued open-label treatment with ELELYSO (9.5 units/kg to 60 units/kg given intravenously every other week) for additional 24 months (total duration of ELELYSO treatment was 33 months).
Baseline Demographics
In Trial 3, patients were 6 to 66 years of age (mean age 42 years, including pediatric patients), 55% were male, 97% were White, and 16% and 84% were Hispanic/Latino and non Hispanic/Latino, respectively.
Efficacy Results
In Trial 3, at baseline, spleen volume was 5.2 (4.5) MN, liver volume was 1.0 (0.3) MN, platelet count was 161,137 (73,387)/mm3, and hemoglobin was 13.5 (1.4) g/dL. Mean (SD) organ volumes and hematologic values remained stable through 9 months of ELELYSO treatment. After 9 months of ELELYSO treatment, spleen volume was 4.8 (4.6) MN, liver volume was 1.0 (0.2) MN, platelet count was 161,167 (80,820)/mm3, and hemoglobin was 13.4 (1.5) g/dL. The ELELYSO dosage remained unchanged in 30 of 31 patients. One patient required a dose increase at Week 24 (from 9.5 units/kg to 19 units/kg) for a platelet count of 92,000/mm3 at Week 22, which subsequently increased to 170,000/mm3 at Month 9.
During the 36‑month period, 18 ELELYSO-treated adult patients maintained stability in clinical parameters (spleen volume, liver volume, platelet count and hemoglobin); however only 10 of 18 adult patients completed 27 months of ELELYSO treatment in the extension trial and only 7 patients had their spleen and liver volumes assessed at 36 months.
During the 33‑month period, the 5 ELELYSO‑treated pediatric patients demonstrated stability in these clinical parameters.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions Including Anaphylaxis
Advise patients and caregivers that life-threatening hypersensitivity reactions, including anaphylaxis may occur with ELELYSO treatment.
Advise patients and caregivers that anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Inform patients and caregivers of the symptoms of life‑threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 200 unit Vial Label
- PRINCIPAL DISPLAY PANEL - 200 unit Vial Carton
-
INGREDIENTS AND APPEARANCE
ELELYSO
taliglucerase alfa injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0069-0106 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALIGLUCERASE ALFA (UNII: 0R4NLX88O4) (TALIGLUCERASE ALFA - UNII:0R4NLX88O4) TALIGLUCERASE ALFA 200 U in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 206.7 mg in 5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.56 mg in 5 mL SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 30.4 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0069-0106-01 1 in 1 CARTON 05/01/2012 1 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022458 05/01/2012 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Registrant - Pfizer Inc (113480771) Establishment Name Address ID/FEI Business Operations Pharmacia & Upjohn Company LLC 618054084 MANUFACTURE(0069-0106) , PACK(0069-0106) , LABEL(0069-0106) Establishment Name Address ID/FEI Business Operations Pfizer Ireland Pharmaceuticals Unlimited Company 985586408 ANALYSIS(0069-0106)