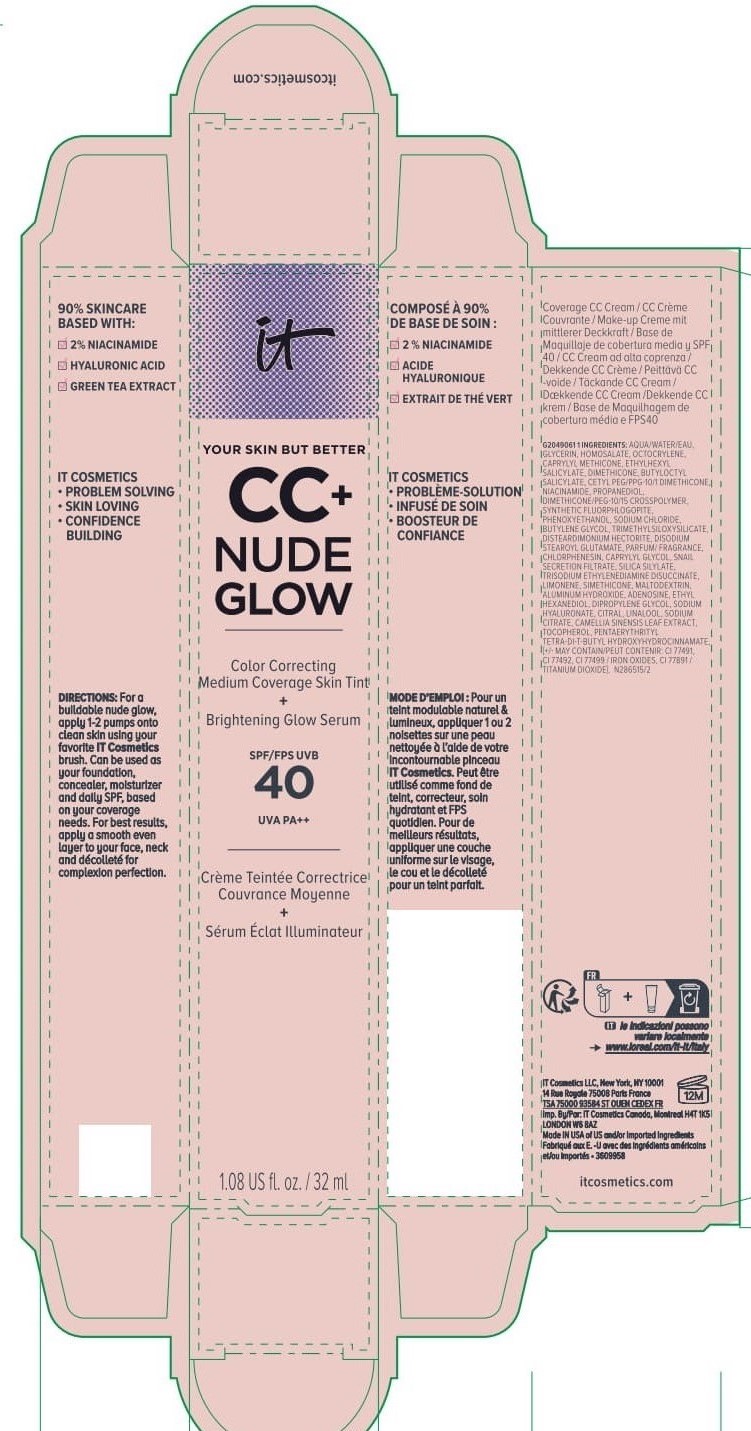
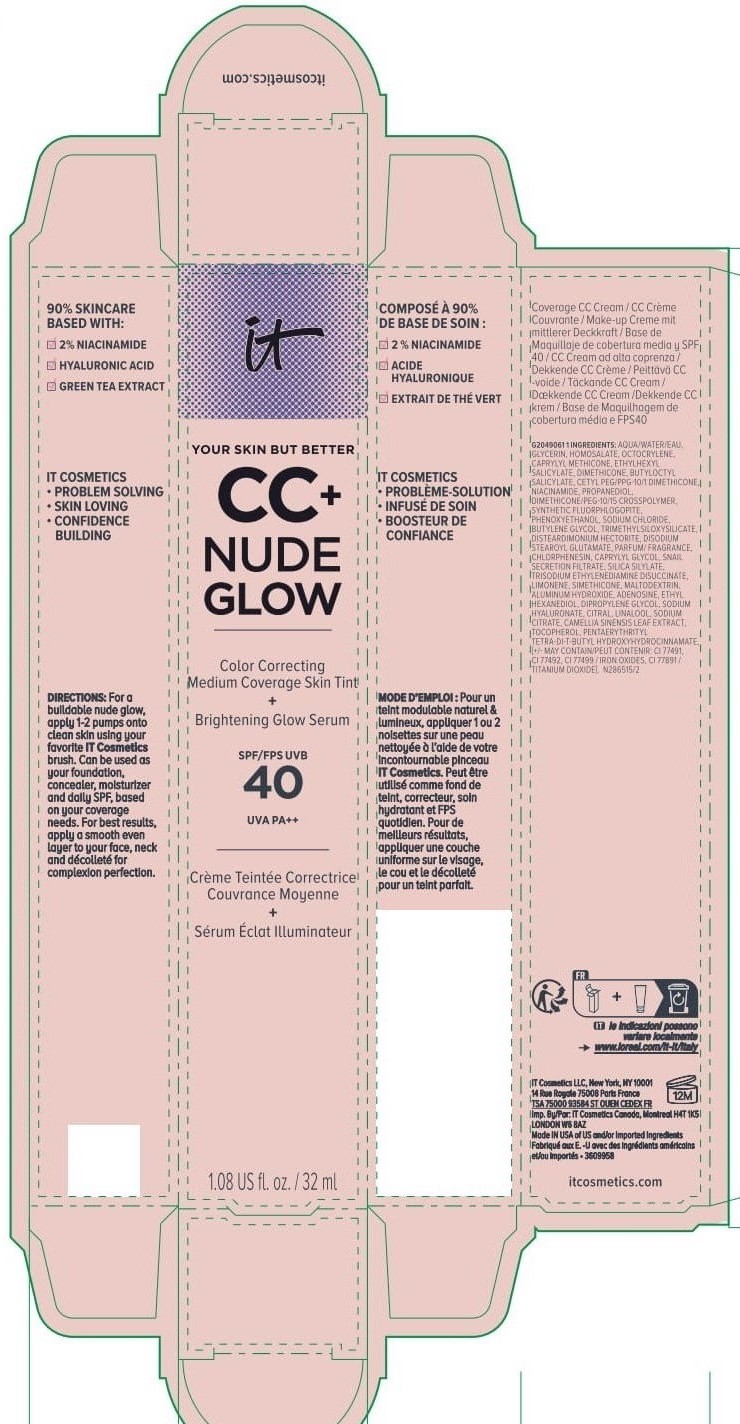
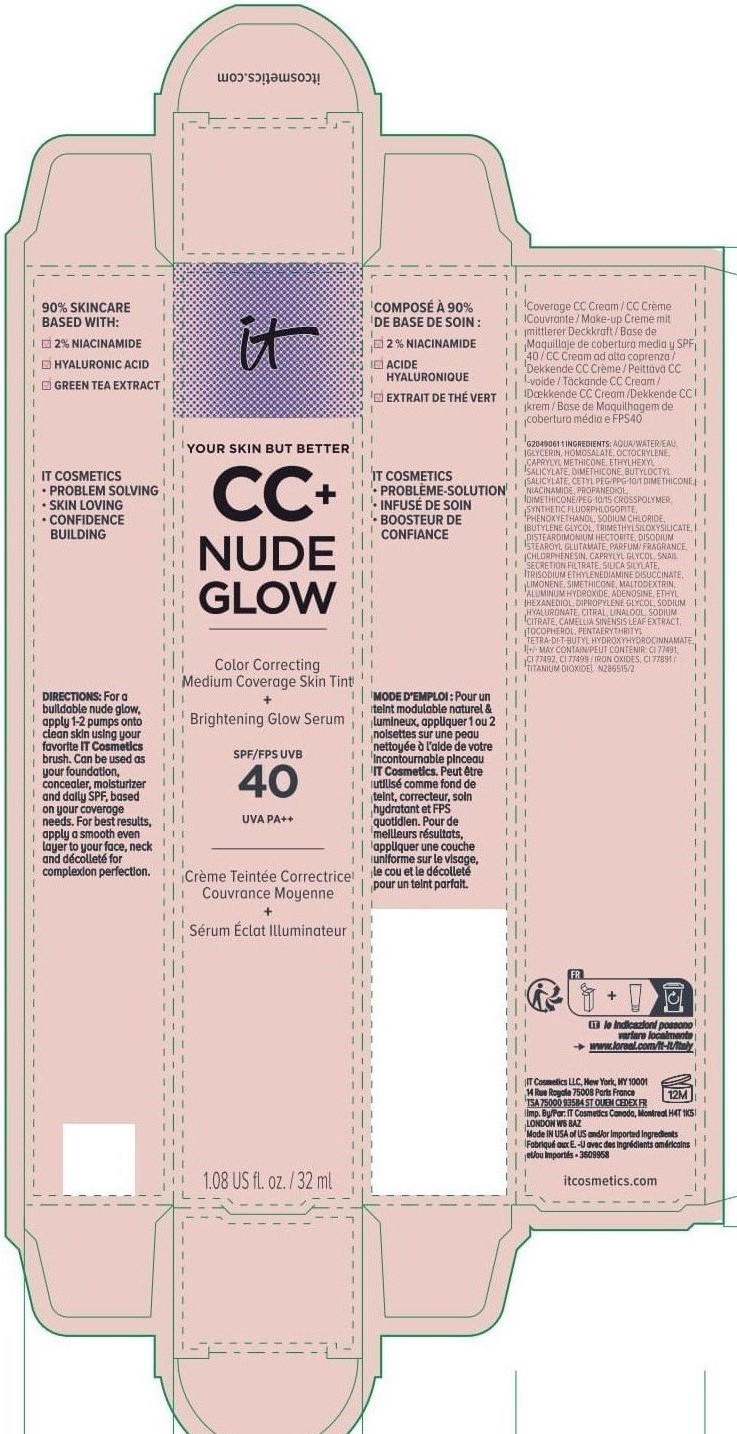
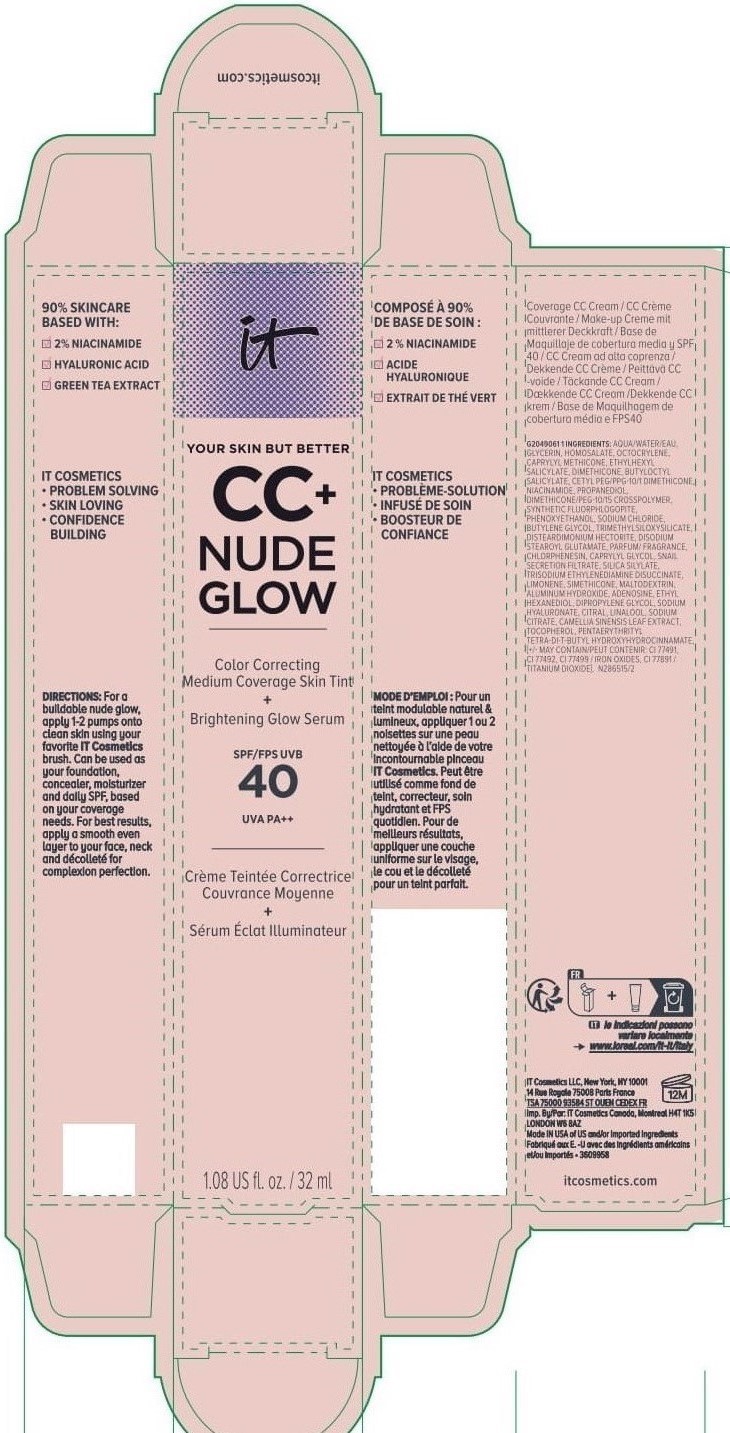
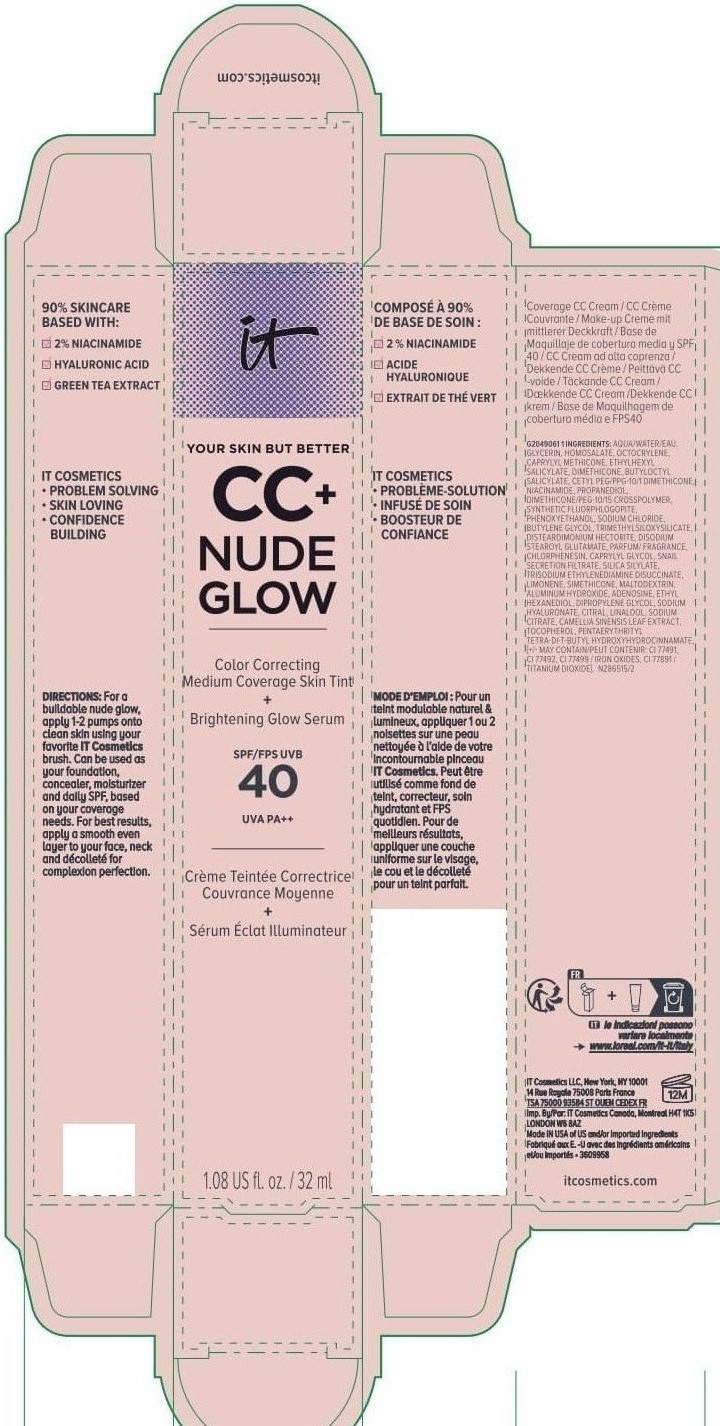
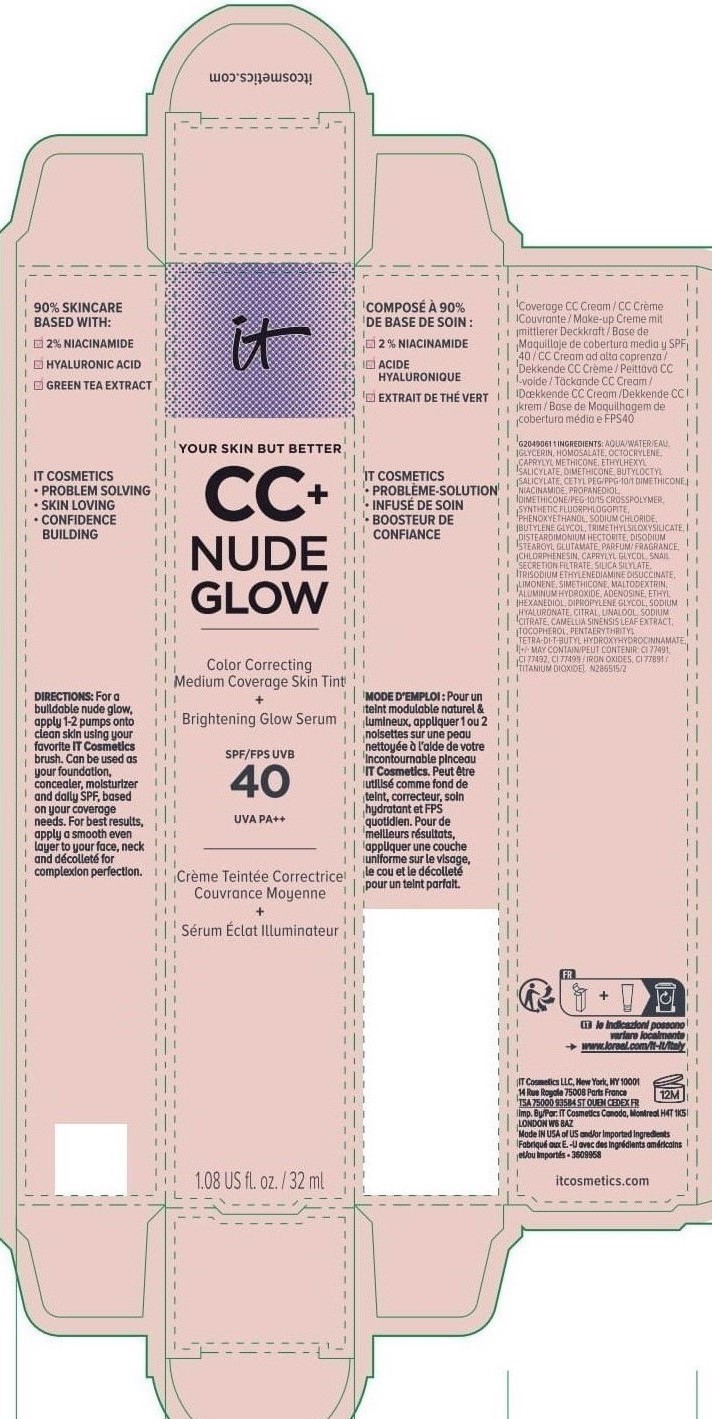
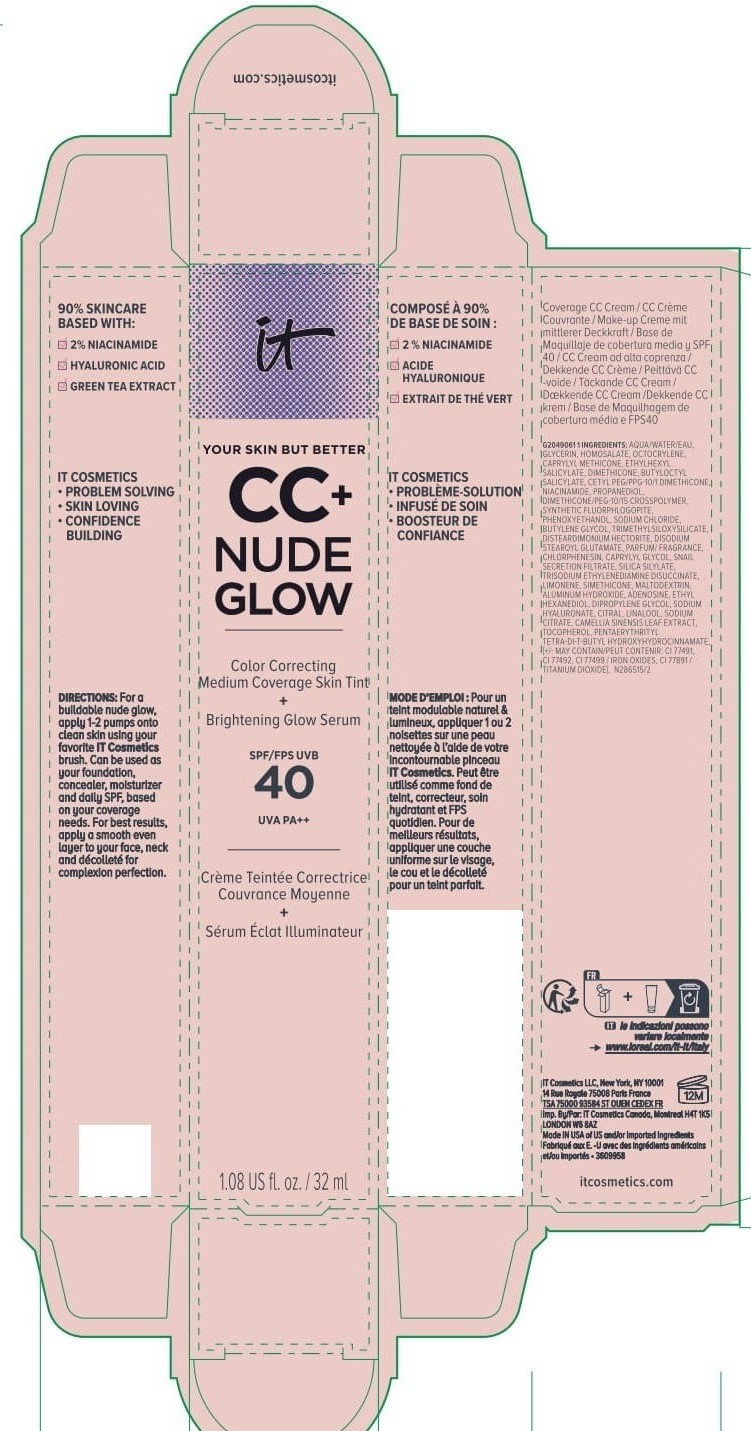
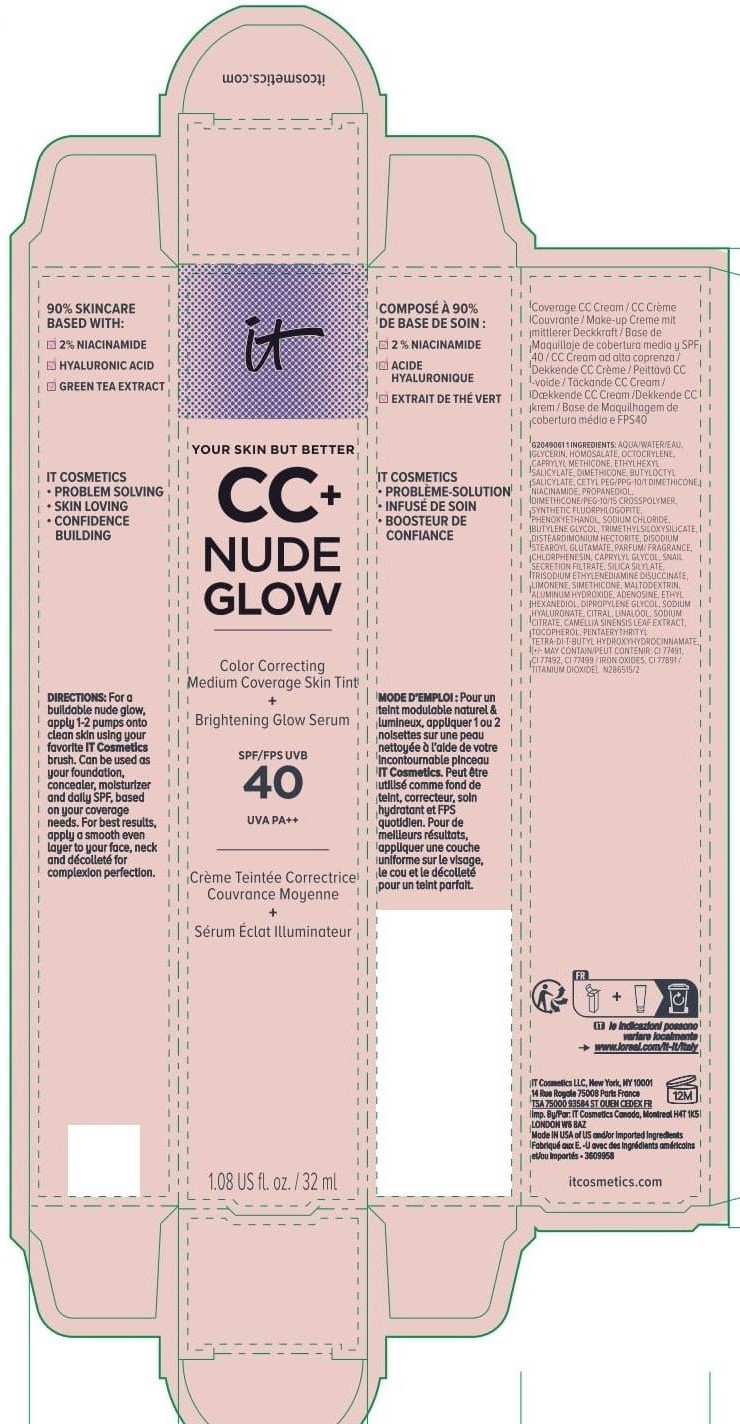
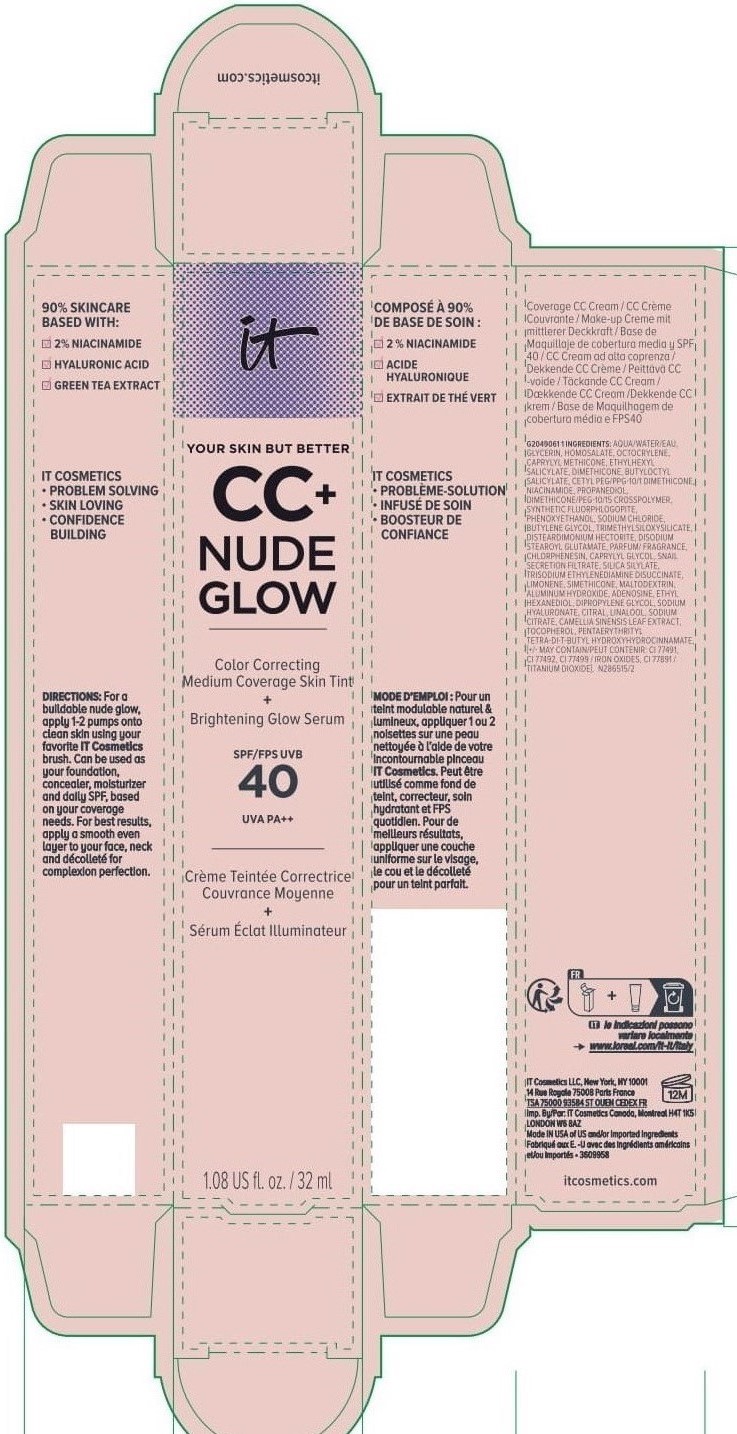
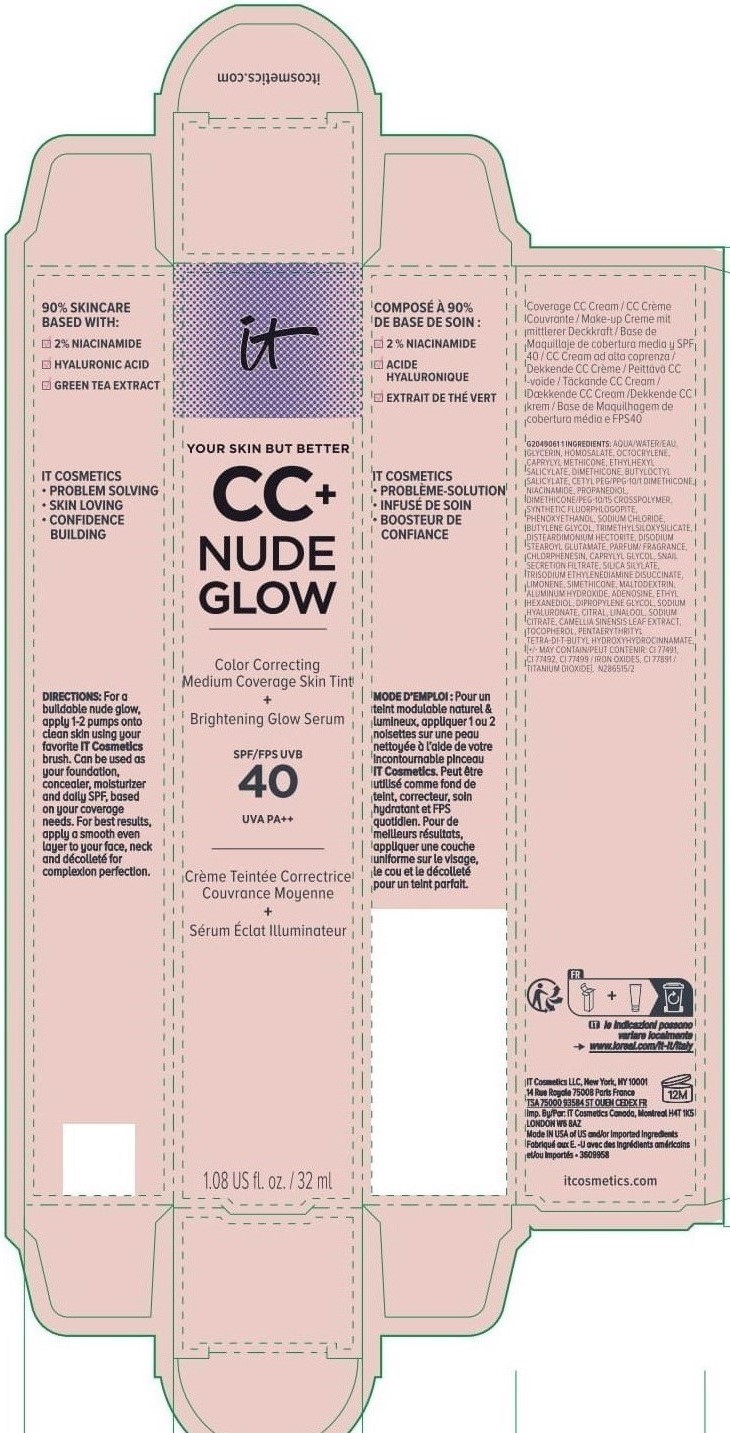
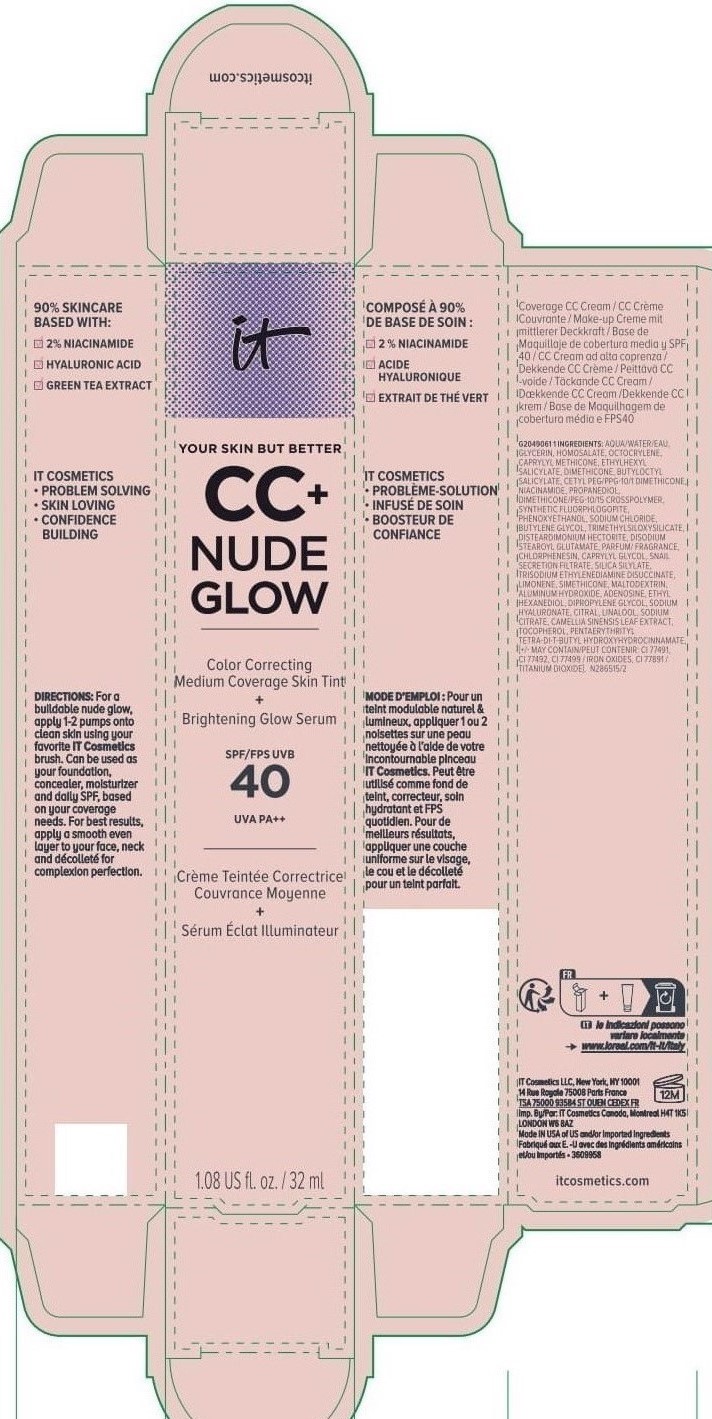
Label: IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL TAN- octocrylene, homosalate, octisalate lotion
IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL MEDIUM (octocrylene, homosalate, octis .......SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - DEEP HONEY- octocrylene, homosalate, octisalate lotion
IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL DEEP- octocrylene, homosalate, octisalate lotion
-
NDC Code(s):
11090-265-01,
11090-266-01,
11090-267-01,
11090-268-01, view more11090-269-01, 11090-270-01, 11090-271-01, 11090-272-01, 11090-273-01, 11090-274-01, 11090-275-01, 11090-276-01, 11090-277-01, 11090-278-01, 11090-279-01, 11090-280-01, 11090-281-01, 11090-282-01, 11090-283-01, 11090-284-01, 11090-285-01, 11090-286-01
- Packager: Beauty Manufacturing Solutions Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
For sunscreen use:
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk,
regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
water, glycerin, caprylyl methicone, dimethicone, butyloctyl salicylate, niacinamide, cetyl PEG/PPG-10/1 dimethicone, propanediol, dimethicone/PEG-10/15 crosspolymer, synthetic fluorphlogopite, butylene glycol, trimethylsiloxysilicate, phenoxyethanol, sodium chloride, disteardimonium hectorite, disodium stearoyl glutamate, fragrance, chlorphenesin, snail secretion filtrate, silica silylate, trisodium ethylenediamine disuccinate, limonene, maltodextrin, aluminum hydroxide, adenosine, dipropylene glycol, ethyl hexanediol, sodium hyaluronate, citral, sodium citrate, linalool, camellia sinensis leaf extract, tocopherol, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate; may contain: titanium dioxide, iron oxides
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL TAN
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-275 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-275-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL MEDIUM
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-273 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-273-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - DEEP
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-282 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-282-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - DEEP BRONZE
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-285 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-285-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - DEEP MOCHA
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-286 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-286-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - RICH HONEY
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-280 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-280-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - TAN
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-276-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - TAN WARM
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-277 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-277-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - RICH
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-279 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-279-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL RICH
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-281 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-281-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - FAIR BEIGE
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-267 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-267-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - FAIR PORCE
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-265 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-265-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - MEDIUM
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-272 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-272-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - MEDIUM TAN
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-274 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-274-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - FAIR LIGHT
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-269 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-269-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - FAIR IVORY
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-266 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) FERRIC OXIDE RED (UNII: 1K09F3G675) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) WATER (UNII: 059QF0KO0R) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-266-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - LIGHT MEDIUM
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-271 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-271-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - LIGHT
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-270-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - FAIR
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-268 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-268-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - TAN RICH
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-278 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-278-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - DEEP HONEY
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-283 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-283-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 IT COSMETICS YOUR SKIN BUT BETTER CC PLUS NUDE GLOW COLOR CORRECTING MEDIUM COVERAGE SKIN TINT BRIGHTENING GLOW SERUM BROAD SPECTRUM SPF 40 SUNSCREEN - NEUTRAL DEEP
octocrylene, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-284 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 56 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 83 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength NIACINAMIDE (UNII: 25X51I8RD4) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CHLORPHENESIN (UNII: I670DAL4SZ) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ADENOSINE (UNII: K72T3FS567) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-284-01 1 in 1 CARTON 03/18/2022 1 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/18/2022 Labeler - Beauty Manufacturing Solutions Corp. (783200723) Registrant - Beauty Manufacturing Solutions Corp. (783200723) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(11090-266, 11090-267, 11090-268, 11090-265, 11090-269, 11090-270, 11090-271, 11090-272, 11090-273, 11090-274, 11090-275, 11090-276, 11090-277, 11090-278, 11090-279, 11090-280, 11090-281, 11090-282, 11090-283, 11090-284, 11090-285, 11090-286)