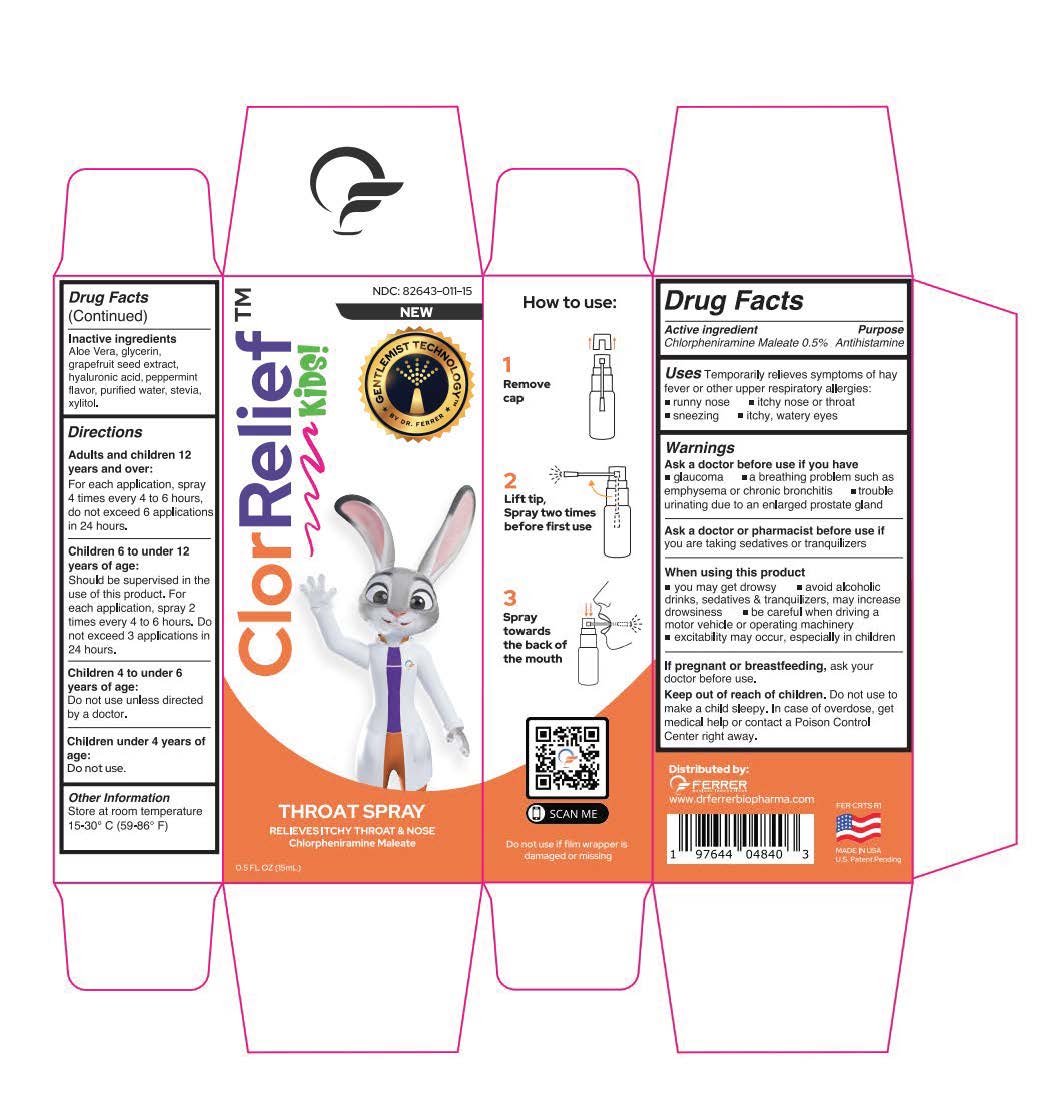
Label: CLORRELIEF KIDS- chlorpheniramine maleate aerosol, spray
- NDC Code(s): 82643-011-15
- Packager: FERRER MEDICAL INNOVATIONS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Uses
-
Warnings
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis trouble urinating due to an enlarged prostate gland
- Inactive ingredients
-
Directions
Adults and children 12 years and over:
For each application, spray 4 times every 4 to 6 hours,
do not exceed 6 applications in 24 hours.
Children 6 to under 12 years of age:
Should be supervised in the use of this product. For each application, spray 2 times every 4 to 6 hours. Do not exceed 3 applications in 24 hours.Children 4 to under 6 years of age:
Do not use unless directed by a doctor.Children under 4 years of age:
Do not use. - Other Information
- ClorRelief Box
-
INGREDIENTS AND APPEARANCE
CLORRELIEF KIDS
chlorpheniramine maleate aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82643-011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA WHOLE (UNII: KIZ4X2EHYX) PEPPERMINT (UNII: V95R5KMY2B) GRAPEFRUIT SEED OIL (UNII: 598D944HOL) HYALURONIC ACID (UNII: S270N0TRQY) STEVIA LEAF (UNII: 6TC6NN0876) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82643-011-15 1 in 1 CARTON 04/24/2023 1 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/21/2023 Labeler - FERRER MEDICAL INNOVATIONS LLC (041608434) Registrant - FERRER MEDICAL INNOVATIONS LLC (041608434) Establishment Name Address ID/FEI Business Operations Xlear Inc. 839884058 manufacture(82643-011)