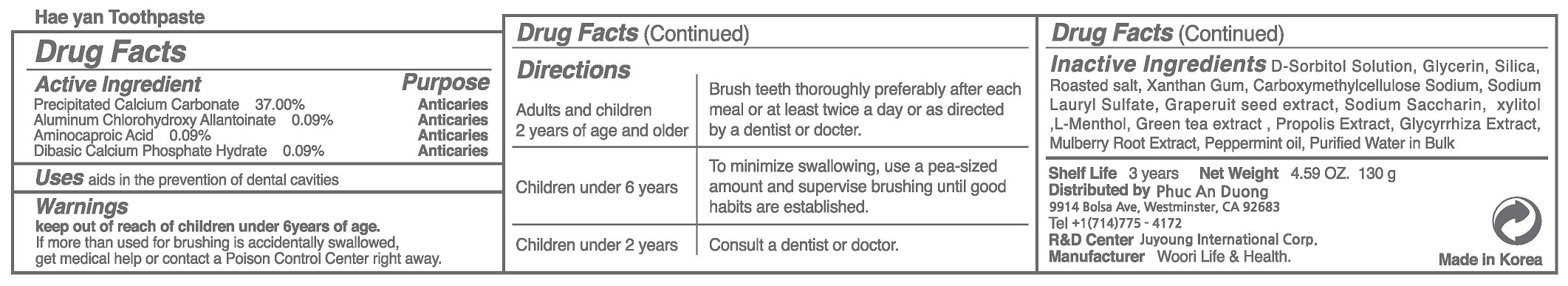
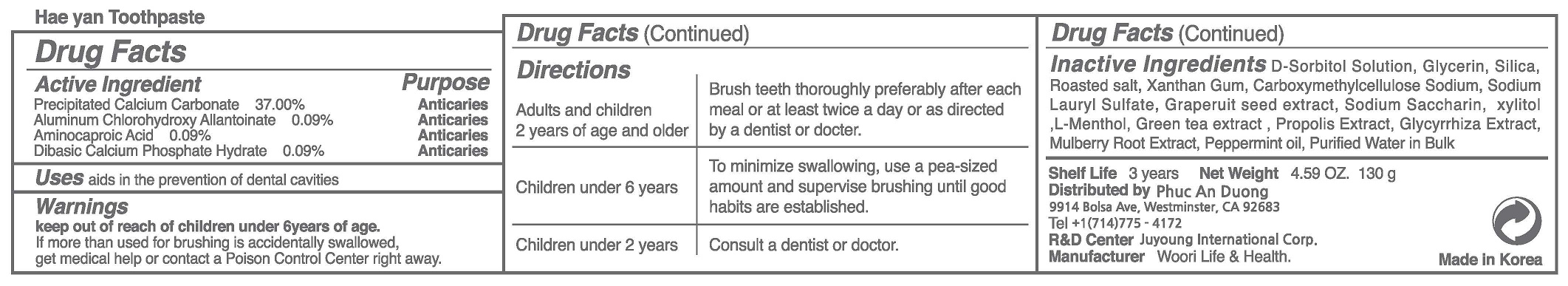
Label: HEA YANTOOTHPASTE- precipitated calcium carbonate, aluminum chlorohydroxy allantoinate, aminocaproic acid, dibasic calcium phosphate hydrate paste, dentifrice
- NDC Code(s): 73065-0008-1
- Packager: WOORI LIFE & HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
To be white and strong, Maintain and clean the mouth, Refresh the oral cavity, Prevention of tooth decay, eliminates bad breat, Enhances the aesthetic effect, Periodontitis (pyorrhea) the prevention, gum disease prevention, Prevention of gingivitis, periodontal disease prevention, gingiva diseage prevention, plaque removal (anti-plaque).
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
(1) Do not swallow, gargle fully after using.
(2) If the use of this paste causes gingiva or oral cavity injury, please stop using and consult a doctor or a dentist.
(3) If childern under 6 years of age use, please apply a pea-sized amount each thime. Do not swallow. Please use under the guidance of the guardian.
(4) If children under 6 years of age accidentally ingest large amounts of the paste, please consult a doctor or a dentist immediately.
(5) Keep out of reach of children under 6 years of age. - INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEA YANTOOTHPASTE
precipitated calcium carbonate, aluminum chlorohydroxy allantoinate, aminocaproic acid, dibasic calcium phosphate hydrate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73065-0008 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMINOCAPROIC ACID (UNII: U6F3787206) (AMINOCAPROIC ACID - UNII:U6F3787206) AMINOCAPROIC ACID 0.09 g in 100 g CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 37 g in 100 g DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) DIBASIC CALCIUM PHOSPHATE DIHYDRATE 0.09 g in 100 g ALCLOXA (UNII: 18B8O9DQA2) (ALCLOXA - UNII:18B8O9DQA2) ALCLOXA 0.09 g in 100 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73065-0008-1 130 g in 1 TUBE; Type 0: Not a Combination Product 04/24/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/24/2023 Labeler - WOORI LIFE & HEALTH (694860803) Registrant - WOORI LIFE & HEALTH (694860803) Establishment Name Address ID/FEI Business Operations WOORI LIFE & HEALTH 694860803 manufacture(73065-0008) , label(73065-0008)